Simulation and Experimental Study of the Multisized Silver Nanoparticles Sintering Process Based on Molecular Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Dynamics Simulation Implementation
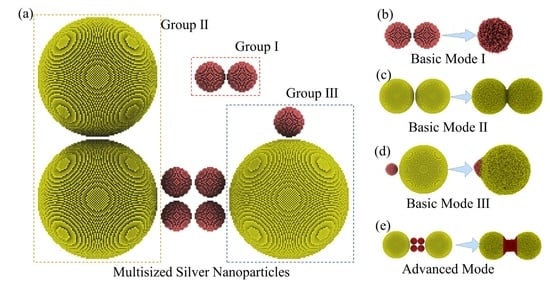
2.2. Modeling and Heating Settings
2.3. Analysis Method of Simulation Results
2.4. Sintering Experiment and Microstructure Characterization
3. Results and Discussion
3.1. Size-Dependent Thermal Stability and Temperature Dependence

3.2. Sintering Stages and Structural Evolution during the Equally Sized Nanoparticle Sintering Process


3.3. Wetting Behavior of Small-Sized Nanoparticles during the Unequally Sized Nanoparticle Sintering Process
3.4. Different Sintering Behaviors Determined by Nanoparticle Sizes in the Multisized Nanoparticle Sintering Process
4. Conclusions
- (1)
- The sintering process of EPs can be divided into three stages: sintering neck formation and fast growth, slow neck growth, and continuous coalescence.
- (2)
- During the sintering process of UPs, small-sized nanoparticles melt, move to the surface of the more significant nanoparticles quickly, and wet the surface of large-sized nanoparticles. This wetting behavior becomes clearer with the increase in size difference between the two nanoparticles.
- (3)
- The sintering behavior of MPs is composed of three basic sintering modes and one advanced sintering mode, including small-sized EPs liquid-phase sintering, large-sized EPs premelting sintering, UPs wetting sintering, and UPs-like structure sintering.
- (4)
- After sintering, large-sized nanoparticles nearly retain their initial shapes and are macroscopically connected by small-sized nanoparticles to form a sintered body.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, H.; Guo, Y.; Huang, S.; Zhang, K.; Yuen, M.M.; Fu, X.; Yu, S.; Sun, R.; Wong, C. One-step preparation of silver hexagonal microsheets as electrically conductive adhesive fillers for printed electronics. ACS Appl. Mater. Interfaces 2015, 7, 13685. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Aroche, A.F.; Schuck, A.; Lamberty, P.; Peter, C.R.; Hasenkamp, W.; Rocha, T.L. Silver nanoparticle conductive inks: Synthesis, characterization, and fabrication of inkjet-printed flexible electrodes. Sci. Rep. 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, Q.; Lu, J.; Zeng, X.; Sun, R.; Wu, J.; Xu, J.; Wong, C. Enhanced thermal conductivity for Ag-deposited alumina sphere/epoxy resin composites through manipulating interfacial thermal resistance. Compos. Part A 2018, 107, 561. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale 2014, 6, 1622. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Shankar, S.; Rhim, J. Melanin-mediated synthesis of silver nanoparticle and its use for the preparation of carrageenan-based antibacterial films. Food Hydrocoll. 2019, 88, 237. [Google Scholar] [CrossRef]
- Li, M.; Xiao, Y.; Zhang, Z.; Yu, J. Bimodal sintered silver nanoparticle paste with ultrahigh thermal conductivity and shear strength for high temperature thermal interface material applications. ACS Appl. Mater. Interfaces 2015, 7, 9157. [Google Scholar] [CrossRef]
- Yang, F.; Hu, B.; Peng, Y.; Hang, C.; Chen, H.; Lee, C.; Wei, J.; Li, M. Ag microflake-reinforced nano-Ag paste with high mechanical reliability for high-temperature applications. J. Mater. Sci. Mater. Electron. 2019, 30, 5526. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Wajahat, M.; Ahn, J.; Pyo, J.; Chang, W.S.; Seol, S.K. 3D printing of highly conductive silver architectures enabled to sinter at low temperatures. Nanoscale 2019, 11, 17682. [Google Scholar] [CrossRef]
- Niittynen, J.; Abbel, R.; Mäntysalo, M.; Perelaer, J.; Schubert, U.S.; Lupo, D. Alternative sintering methods compared to conventional thermal sintering for inkjet printed silver nanoparticle ink. Thin Solid Films 2014, 556, 452. [Google Scholar] [CrossRef]
- Vandevenne, G.; Marchal, W.; Verboven, I.; Drijkoningen, J.; D’Haen, J.; van Bael, M.K.; Hardy, A.; Deferme, W. A study on the thermal sintering process of silver nanoparticle inkjet inks to achieve smooth and highly conducting silver layers. Phys. Status Solidi A 2016, 213, 1403. [Google Scholar] [CrossRef]
- Sanchez-Romaguera, V.; Wünscher, S.; Turki, B.M.; Abbel, R.; Barbosa, S.; Tate, D.J.; Oyeka, D.; Batchelor, J.C.; Parker, E.A.; Schubert, U.S.; et al. Inkjet printed paper based frequency selective surfaces and skin mounted RFID tags: The interrelation between silver nanoparticle ink, paper substrate and low temperature sintering technique. J. Mater. Chem. C 2015, 3, 2132. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.Y.; Xu, C.X.; Hu, A.M.; Oakes, K.D.; Sheng, F.Y.; Shi, Z.L.; Dai, J.; Jin, Z.L. Sintering Dynamics and Thermal Stability of Novel Configurations of Ag Clusters. Phys. Chem. Solids 2012, 73, 1350. [Google Scholar] [CrossRef]
- Buesser, B.; Grohn, A.J.; Pratsinis, S.E. Sintering Rate and Mechanism of TiO Nanoparticles by Molecular Dynamics. J. Mater. Chem. C 2011, 115, 11030. [Google Scholar]
- Cheng, B.; Ngan, A.H. The Sintering and Densification Behaviour of Many Copper Nanoparticles: A Molecular Dynamics Study. Comput. Mater. Sci. 2013, 74, 1. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Gan, Y.; Zhang, Y.; Chen, J.K. Molecular Dynamics Simulation of Neck Growth in Laser Sintering of Different-Sized Gold Nanoparticles under Different Heating Rates. Appl. Phys. A 2012, 106, 725. [Google Scholar] [CrossRef]
- Grammatikopoulos, P.; Cassidy, C.; Singh, V.; Benelmekki, M.; Sowwan, M. Coalescence Behaviour of Amorphous and Crystalline Tantalum Nanoparticles: A Molecular Dynamics Study. J. Mater. Sci. 2014, 49, 3890. [Google Scholar] [CrossRef]
- Moitra, A.; Kim, S.; Kim, S.G.; Park, S.J.; German, R.M.; Horstemeyer, M.F. Investigation on sintering mechanism of nanoscale tungsten powder based on atomistic simulation. Acta Materialia 2010, 58, 3939. [Google Scholar] [CrossRef]
- Hussein, O.; Alghalayini, M.; Dillon, S.J.; Abdeljawad, F. Unraveling the Role of Grain Boundary Anisotropy in Sintering: Implications for Nanoscale Manufacturing. ACS Appl. Nano Mater. 2021, 4, 8039. [Google Scholar] [CrossRef]
- Sestito, J.M.; Abdeljawad, F.; Harris, T.A.; Wang, Y.; Roach, A. An atomistic simulation study of nanoscale sintering: The role of grain boundary misorientation. Comput. Mater. Sci. 2019, 165, 180. [Google Scholar] [CrossRef]
- Seong, Y.; Kim, Y.; German, R.; Kim, S.; Kim, S.G.; Kim, S.J.; Kim, H.J.; Park, S.J. Dominant mechanisms of the sintering of copper nano-powders depending on the crystal misalignment. Comput. Mater. Sci. 2016, 123, 164. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J. Sintering phenomena and mechanical strength of nickel based materials in direct metal laser sintering process—A molecular dynamics study. J. Mater. Res. 2016, 31, 2233. [Google Scholar] [CrossRef]
- Hwang, H.; Malhotra, R. Shape-tuned junction resistivity and self-damping dynamics in intense pulsed light sintering of silver nanostructure films. ACS Appl. Mater. Interfaces 2018, 11, 3536. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Davidchack, R.L.; Pan, J. AMolecular Dynamics Study of Sintering between Nanoparticles. Comput. Mater. Sci. 2009, 45, 247. [Google Scholar] [CrossRef]
- Daw, M.S.; Baskes, M.I. Embedded-Atom Method: Derivation and Application to Impurities, Surfaces, and Other Defects in Metals. Phys. Rev. B 1984, 29, 6443. [Google Scholar] [CrossRef] [Green Version]
- Rassoulinejad-Mousavi, S.M.; Zhang, Y. Interatomic potentials transferability for molecular simulations: A comparative study for platinum, gold and silver. Sci. Rep. 2018, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Foiles, S.M.; Baskes, M.I.; Daw, S. Embedded-atom-method functions for the fcc metals Cu, Ag, Au, Ni, Pd, Pt, and their alloys. Phys. Rev. B 1986, 33, 7983. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1. [Google Scholar] [CrossRef] [Green Version]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J Chem. Phys. 1984, 81, 511. [Google Scholar] [CrossRef] [Green Version]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef] [Green Version]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Modell. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Wang, J.; Shin, S.; Hu, A. Geometrical effects on sintering dynamics of Cu–Ag core–shell nanoparticles. Molecular Dynamics Simulation of the Melting Processes of Core−Shell and Pure Nanoparticles. J. Mater. Chem. C 2016, 120, 17791. [Google Scholar]
- Tamura, Y.; Arai, N. Molecular Dynamics Simulation of the Melting Processes of Core−Shell and Pure Nanoparticles. Mol. Simul. 2015, 41, 905. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, J.; Zhang, H.; Zhao, F. Molecular Dynamics Simulations on the Melting, Crystallization, and Energetic Reaction Behaviors of Al/Cu Core-Shell Nanoparticles. J. Appl. Phys. 2013, 114, 084310. [Google Scholar] [CrossRef]
- Song, P.; Wen, D. Molecular Dynamics Simulation of the Sintering of Metallic Nanoparticles. J. Nanopart. Res. 2010, 12, 823. [Google Scholar] [CrossRef]
- Ackland, G.J.; Jones, A.P. Applications of Local Crystal Structure Measures in Experiment and Simulation. Phys. Rev. B 2006, 73, 054104. [Google Scholar] [CrossRef]
- Lechner, W.; Dellago, C. Accurate Determination of Crystal Structures Based on Averaged Local Bond Order Parameters. J. Chem. Phys. 2008, 129, 114707. [Google Scholar] [CrossRef]
- Stukowski, A. Structure Identification Methods for Atomistic Simulations of Crystalline Materials. Modell. Simul. Mater. Sci. Eng. 2012, 20, 045021. [Google Scholar] [CrossRef]
- Feng, D.; Feng, Y.; Yuan, S.; Zhang, X.; Wang, G. Melting behavior of Ag nanoparticles and their clusters. Appl. Therm. Eng. 2017, 111, 1457. [Google Scholar] [CrossRef]

















| Model | SP | EPs | UPs | MPs |
|---|---|---|---|---|
| Diameter (nm) | 5–20 | Equally sized | Unequally sized | Multisized |
| Edge-to-edge distance (nm) | - | 0.5 | 0.5 | 0.5 |
| Atom numbers | 3805–244,961 | 7610–489,922 | 248,766; 260,644 | 505,142 |
| Morphology 1 |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, M.; Liu, T.; Xiao, X.; Li, G.; Liao, W. Simulation and Experimental Study of the Multisized Silver Nanoparticles Sintering Process Based on Molecular Dynamics. Nanomaterials 2022, 12, 1030. https://doi.org/10.3390/nano12061030
Gu M, Liu T, Xiao X, Li G, Liao W. Simulation and Experimental Study of the Multisized Silver Nanoparticles Sintering Process Based on Molecular Dynamics. Nanomaterials. 2022; 12(6):1030. https://doi.org/10.3390/nano12061030
Chicago/Turabian StyleGu, Mingfei, Tingting Liu, Xingzhi Xiao, Gang Li, and Wenhe Liao. 2022. "Simulation and Experimental Study of the Multisized Silver Nanoparticles Sintering Process Based on Molecular Dynamics" Nanomaterials 12, no. 6: 1030. https://doi.org/10.3390/nano12061030