Carbon Dot-Decorated Graphite Carbon Nitride Composites for Enhanced Solid-Phase Microextraction of Chlorobenzenes from Water
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Apparatus
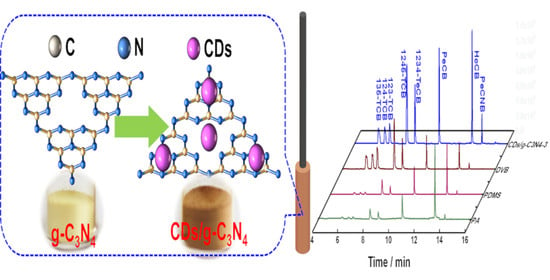
2.3. Synthesis of CDs/g-C3N4 Composites
2.4. Fabrication of SPME Fiber Based on CDs/g-C3N4 Composites
2.5. SPME Procedure and Real Samples Analysis
3. Results and Discussion
3.1. Characterizations of CDs/g-C3N4 Composites
3.2. SPME Performance of CDs/g-C3N4 Composites
3.3. Optimization of SPME Conditions
3.4. Method Performance
3.5. Real Water Samples Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Zhou, B.; Liu, Y.; Liu, J.; Hu, L.; Tang, Y.; Wang, M. In-situ regulation of acid sites on Mn-based perovskite@mullite composite for promoting catalytic oxidation of chlorobenzene. J. Colloid. Interf. Sci. 2022, 606, 1866–1873. [Google Scholar] [CrossRef]
- Zhu, Q.; Yan, J.; Dai, Q.; Wu, Q.; Cai, Y.; Wu, J.; Wang, X.; Zhan, W. Ethylene glycol assisted synthesis of hierarchical Fe-ZSM-5 nanorods assembled microsphere for adsorption Fenton degradation of chlorobenzene. J. Hazard. Mater. 2020, 385, 121581. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, H.; Li, Y.; He, X.; Chen, L.; Zhang, Y. Progress in application of functionalized magnetic nanomaterials for sample pretreatment. Chin. J. Chromatogr. 2020, 38, 2–13. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Reyes-Garces, N.; Gionfriddo, E.; Gmez-Rios, G.A.; Alam, M.N.; Boyaci, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in solid phase microextraction and perspective on future directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Wang, W.; Zhang, S.; Wang, C.; Zhou, J.; Wang, Z. Solid phase microextraction of polycyclic aromatic hydrocarbons by using an etched stainless-steel fiber coated with a covalent organic framework. Microchim. Acta. 2019, 186, 145. [Google Scholar] [CrossRef]
- Guo, Y.; He, X.; Huang, C.; Chen, H.; Lu, Q.; Zhang, L. Metal–organic framework-derived nitrogen-doped carbon nanotube cages as efficient adsorbents for solid-phase microextraction of polychlorinated biphenyls. Anal. Chim. Acta 2020, 1095, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, J.; Zhang, X.; Han, L.; Qin, P.; Tian, S.; Zhou, Q.; Zhang, X.; Lu, M. Preparation of Al-doped mesoporous crystalline material-41 as fiber coating material for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons from human urine. J. Chromatogr. A 2020, 1626, 461354. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.; Shi, Y.; Peng, X.; Kuang, Y.; Zhou, S.; Zheng, J.; Yang, X.; Ouyang, G. Polystyrene-based nanospheres with controllable microstructures for exceptional solid phase microextraction of organic pollutants. Chem. Eng. J. 2022, 428. [Google Scholar] [CrossRef]
- Song, X.; Wang, R.; Wang, X.; Han, H.; Qiao, Z.; Sun, X.; Ji, W. An amine-functionalized olefin-linked covalent organic framework used for the solid-phase microextraction of legacy and emerging per- and polyfluoroalkyl substances in fish. J. Hazard. Mater. 2022, 423, 127226. [Google Scholar] [CrossRef]
- Cheng, H.; Song, Y.; Bian, Y.; Ji, R.; Wang, F.; Gu, C.; Yang, X.; Ye, M.; Ouyang, G.; Jiang, X. Meso-/microporous carbon as an adsorbent for enhanced performance in solid-phase microextraction of chlorobenzenes. Sci. Total Environ. 2019, 681, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, P.; Das, T.K.; Ghosh, S.; Das, S.; Bose, M.; Mondal, M.; Das, A.K.; Das, N.C. Acoustic cavitation assisted destratified clay tactoid reinforced in situ elastomer-mimetic semi-IPN hydrogel for catalytic and bactericidal application. Ultrason. Sonochem. 2020, 60, 104797. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Saha, A.; Noked, M.; Margel, S.; Gedanken, A. Carbon-dots-initiated photopolymerization: An in situ synthetic approach for MXene/Poly(norepinephrine)/copper hybrid and its application for mitigating water pollution. ACS Appl. Mater. Inter. 2021, 13, 31038–31050. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, J.; Yang, S.; Lin, X.; Guo, F.; Shi, J. Fabrication of a ternary carbon dots/CoO/g-C3N4 nanocomposite photocatalyst with enhanced visible-light-driven photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2020, 95, 2129–2138. [Google Scholar] [CrossRef]
- Dadigala, R.; Bandi, R.; Gangapuram, B.R.; Guttena, V. Carbon dots and Ag nanoparticles decorated g-C3N4 nanosheets for enhanced organic pollutants degradation under sunlight irradiation. J. Photochem. Photobiol. A Chem. 2017, 342, 42–52. [Google Scholar] [CrossRef]
- Feng, H.; Qian, Z. Functional carbonquantum dots: A versatile platform for chemosensing and biosensing. Chem. Rec. 2018, 18, 491–505. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharmaceut. 2019, 564, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gong, Z.; Tang, W.; Row, K.H.; Qiu, H. Carbon dots in sample preparation and chromatographic separation: Recent advances and future prospects. Trac-Trend. Anal. Chem. 2021, 134, 116135. [Google Scholar] [CrossRef]
- Li, Y.-K.; Yang, T.; Chen, M.-L.; Wang, J.-H. Supported carbon dots serve as high-performance adsorbent for the retention of trace cadmium. Talanta 2018, 180, 18–24. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, Y.; Lin, Y.; Zhou, J. Loading controlled magnetic carbon dots for microwave-assisted solid-phase extraction: Preparation, extraction evaluation and applications in environmental aqueous samples. J. Sep. Sci. 2018, 41, 3622–3630. [Google Scholar] [CrossRef]
- Cai, T.; Zhang, H.; Rahman, A.F.M.M.; Shi, Y.-P.; Qiu, H. Silica grafted with silanized carbon dots as a nano-on-micro packing material with enhanced hydrophilic selectivity. Microchim. Acta 2017, 184, 2629–2636. [Google Scholar] [CrossRef]
- Cai, T.; Zhang, H.; Chen, J.; Li, Z.; Qiu, H. Polyethyleneimine-functionalized carbon dots and their precursor co-immobilized on silica for hydrophilic interaction chromatography. J. Chromatogr. A 2019, 1597, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Ha, W.; Chen, J.; Qi, H.-Y.; Shi, Y.-P. Advances and applications of graphitic carbon nitride as sorbent in analytical chemistry for sample pretreatment: A review. Trac-Trend. Anal. Chem. 2016, 84, 12–21. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Zhu, W.; Qin, P.; Lu, M.; Zhang, X.; Miao, Y.; Cai, Z. Mesoporous graphitic carbon nitride@NiCo2O4 nanocomposite as a solid phase microextraction coating for sensitive determination of environmental pollutants in human serum samples. Chem. Commun. 2019, 55, 10019–10022. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, P.; Zhang, J.; Li, W.; Zhu, J.; Lu, M.; Cai, Z. Fabrication of nanoscale graphitic carbon nitride/copper oxide hybrid composites coated solid-phase microextraction fibers coupled with gas chromatography for determination of polycyclic aromatic hydrocarbons. J. Chromatogr. A 2018, 1570, 47–55. [Google Scholar] [CrossRef]
- Han, L.; Yang, Y.; Zhang, J.; Guo, J.; Lu, M. Recent advances in graphitic carbon nitride materials for sample pretreatment. Chin. J. Chromatogr. 2020, 38, 28–35. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Han, B.; Hu, X.; Yang, F.; Xu, Z.; Li, Y.; Jia, S.; Li, Z.; et al. Carbon quantum dot implanted graphite carbon nitride nanotubes: Excellent charge separation and enhanced photocatalytic hydrogen evolution. Angew. Chem. Int. Edit. 2018, 57, 5765–5771. [Google Scholar] [CrossRef]
- Dillip, G.R.; Sreekanth, T.V.M.; Joo, S.W. Tailoring the bandgap of N-rich graphitic carbon nitride for enhanced photocatalytic activity. Ceram. Int. 2017, 43, 6437–6445. [Google Scholar] [CrossRef]
- Xu, S.; Dong, P.; Qin, M.; Liu, H.; Long, A.; Chen, C.; Feng, S.; Wu, H. Core-shell structured Fe2O3/CeO2@MnO2 microspheres with abundant surface oxygen for sensitive solid-phase microextraction of polycyclic aromatic hydrocarbons from water. Microchim. Acta 2021, 188, 116135. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Q.; Wang, C.; Xiao, L.; Feng, S.; Li, N.; Chen, C.-P. Three-dimensional pompon-like Au/ZnO porous microspheres as solid phase microextraction coating for determination of volatile fatty acids from foot odor. Talanta 2020, 209, 120519. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Li, M.; Qin, P.; Li, D.; Zhou, Q.; Lu, M.; Cai, Z. Nitrogen-rich carbon nitride as solid-phase microextraction fiber coating for high-efficient pretreatment of polychlorinated biphenyls from environmental samples. J. Chromatogr. A 2021, 1659, 462655. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Geng, F.; Guo, L.-H.; Wan, B.; Yang, Y. Carbon dots decorated graphitic carbon nitride as an efficient metal-free photocatalyst for phenol degradation. Appl. Catal. B Environ. 2016, 180, 656–662. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Pan, H.; Liu, Y.; Xu, S.; Cao, S. In situ fabrication of CDs/g-C3N4 hybrids with enhanced interface connection via calcination of the precursors for photocatalytic H2 evolution. Int. J. Hydrogen Energy 2018, 43, 91–99. [Google Scholar] [CrossRef]
- Groenewolt, M.; Antonietti, M. Synthesis of g-C3N4 nanoparticles in mesoporous silica host matrices. Adv. Mater. 2005, 17, 1789–1792. [Google Scholar] [CrossRef]
- Li, K.; Su, F.-Y.; Zhang, W.-D. Modification of g-C3N4 nanosheets by carbon quantum dots for highly efficient photocatalytic generation of hydrogen. Appl. Surf. Sci. 2016, 375, 110–117. [Google Scholar] [CrossRef]
- Jian, X.; Liu, X.; Yang, H.-M.; Li, J.-G.; Song, X.-L.; Dai, H.-Y.; Liang, Z.-H. Construction of carbon quantum dots/proton-functionalized graphitic carbon nitride nanocomposite via electrostatic self-assembly strategy and its application. Appl. Surf. Sci. 2016, 370, 514–521. [Google Scholar] [CrossRef]
- Tripathi, A.; Narayanan, S. Potassium doped graphitic carbon nitride with extended optical absorbance for solar light driven photocatalysis. Appl. Surf. Sci. 2019, 479, 1–11. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, C.; Zhang, L.; Hou, Y.; Wang, S.; Dong, P.; Lin, F.; Wang, Y. Facile two-step synthesis of porous carbon nitride with enhanced photocatalytic activity using a soft template. Acs. Sustain. Chem. Eng. 2019, 7, 3866–3874. [Google Scholar] [CrossRef]
- Cui, L.; Liu, Y.; Fang, X.; Yin, C.; Li, S.; Sun, D.; Kang, S. Scalable and clean exfoliation of graphitic carbon nitride in NaClO solution: Enriched surface active sites for enhanced photocatalytic H2 evolution. Green Chem. 2018, 20, 1354–1361. [Google Scholar] [CrossRef]
- Feng, Z.; Huang, C.; Guo, Y.; Liu, W.; Zhang, L. Graphitic carbon nitride derivative with large mesopores as sorbent for solid-phase microextraction of polycyclic aromatic hydrocarbons. Talanta 2020, 209, 120541. [Google Scholar] [CrossRef]
- Xu, L.; Huang, S.; Liu, Y.; Wei, S.; Chen, G.; Gong, Z.; Ouyang, G. Hollow carbon nanobubbles-coated solid-phase microextraction fibers for the sensitive detection of organic pollutants. Anal. Chim. Acta 2020, 1097, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, L.; Zheng, J.; Jiang, R.; Zhu, F.; Luan, T.; Ouyang, G. Mesoporous TiO2 nanoparticles for highly sensitive solid-phase microextraction of organochlorine pesticides. Anal. Chim. Acta 2015, 878, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Shi, X.; Wu, J.; Huang, X. Graphene reinforced multiple monolithic fiber solid-phase microextraction of phenoxyacetic acid herbicides in complex samples. Talanta 2019, 191, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, F.; Zhang, R.; Wang, Z.; Du, X. A new strategy for electrochemical fabrication of manganese dioxide coatings based on silica nanoparticles deposited on titanium fibers for selective and highly efficient solid-phase microextraction. New J. Chem. 2019, 43, 5055–5064. [Google Scholar] [CrossRef]
- Zhao, F.; Lu, S.; Du, W.; Zeng, B. Ionic liquid-based headspace single-drop microextraction coupled to gas chromatography for the determination of chlorobenzene derivatives. Microchim. Acta 2009, 165, 29–33. [Google Scholar] [CrossRef]
- Ma, X.; Ma, J. Determination of trace amounts of chlorobenzenes in water using membrane-supported headspace single-drop microextraction and gas chromatography–mass spectrometry. J. Anal. Chem. 2017, 72, 890–896. [Google Scholar] [CrossRef]
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. A simple device for headspace sorptive extraction prior to gas chromatography–mass spectrometry analysis. Talanta 2019, 195, 796–799. [Google Scholar] [CrossRef]
- Cheng, H.; Song, Y.; Bian, Y.; Wang, F.; Ji, R.; He, W.; Gu, C.; Ouyang, G.; Jiang, X. A nanoporous carbon material coated onto steel wires for solid-phase microextraction of chlorobenzenes prior to their quantitation by gas chromatography. Microchim. Acta 2017, 185, 56. [Google Scholar] [CrossRef]
- Ghasemi, E.; Sillanpää, M. Optimization of headspace solid phase microextraction based on nano-structured ZnO combined with gas chromatography–mass spectrometry for preconcentration and determination of ultra-traces of chlorobenzenes in environmental samples. Talanta 2014, 130, 322–327. [Google Scholar] [CrossRef]
- Ji, R.; Wu, Y.; Bian, Y.; Song, Y.; Sun, Q.; Jiang, X.; Zhang, L.; Han, J.; Cheng, H. Nitrogen-doped porous biochar derived from marine algae for efficient solid-phase microextraction of chlorobenzenes from aqueous solution. J. Hazard. Mater. 2021, 407, 124785. [Google Scholar] [CrossRef]





| Analytes | Linear Ranges (ng L−1) | R2 | LODs (ng L−1) | LOQs (ng L−1) | RSDs (%) | |
|---|---|---|---|---|---|---|
| One Fiber (n = 7) | Fiber-to-Fiber (n = 5) | |||||
| 135-TCB | 0.25–2500 | 0.9989 | 0.003 | 0.010 | 8.0 | 9.1 |
| 124-TCB | 0.25–2500 | 0.9974 | 0.086 | 0.288 | 9.7 | 12.6 |
| 123-TCB | 0.25–2500 | 0.9948 | 0.033 | 0.108 | 9.4 | 10.3 |
| 1245-TeCB | 0.25–2500 | 0.9865 | 0.007 | 0.024 | 5.3 | 7.5 |
| 1234-TeCB | 0.25–2500 | 0.9822 | 0.016 | 0.053 | 7.7 | 9.5 |
| PeCB | 0.25–2500 | 0.9933 | 0.005 | 0.016 | 8.5 | 8.9 |
| HeCB | 0.25–2500 | 0.9996 | 0.002 | 0.007 | 6.1 | 8.2 |
| PeCNB | 0.25–2500 | 0.9945 | 0.024 | 0.083 | 7.2 | 9.8 |
| Methods | Sorbents | Linear Range (ng L−1) | LODs (ng L−1) | LOQs (ng L−1) | References |
|---|---|---|---|---|---|
| SDME/GC-FID | 1-Octyl-3-Methylimidazolium hexafluorophosphate | 1000–1 × 107 | 100–500 | – | [45] |
| SDME/GC-MS | Toluene | 500–50,000 | 10–50 | 40–440 | [46] |
| HSSE/GC-MS | PDMS coated stir bar | 10–200 | 0.4–1.4 | 1.4–4.7 | [47] |
| SPME/GC-ECD | Nanoporous carbon | 10–500 2.5–100 | 0.10–1.03 | – | [48] |
| HS-SPME/GC-MS | Nano-structured ZnO | 0.05–1000 | 0.01–0.1 | – | [49] |
| SPME/GC-ECD | Nitrogen/Porous biochar | 1–1000 | 0.007–0.079 | 0.023–0.261 | [50] |
| SPME/GC-MS | CDs/g-C3N4 | 0.25–2500 | 0.002–0.086 | 0.007–0.288 | This work |
| Analytes | 135-TCB | 124-TCB | 123-TCB | 1245-TeCB | 1234-TeCB | PeCB | HeCB | PeCNB | |
|---|---|---|---|---|---|---|---|---|---|
| 1# | Found (ng L−1) | ND | ND | 1.7 | ND | ND | ND | ND | ND |
| RSDs (%, n = 3) | - | - | 0.8 | - | - | - | - | - | |
| Recoveries (%, spiked with 1 ng L−1) | 105.1 | 75.5 | 88.8 | 94.5 | 93.8 | 80.3 | 101.1 | 83.4 | |
| 2# | Found (ng L−1) | 154.0 | ND | 11.7 | ND | ND | ND | 57.6 | ND |
| RSDs (%, n = 3) | 11.4 | - | 13.9 | - | - | - | 20.6 | - | |
| Recoveries (%, spiked with 10 ng L−1) | 108.5 | 105.5 | 109.1 | 84.4 | 84.2 | 122.3 | 93.5 | 75.5 | |
| 3# | Found (ng L−1) | 60.4 | ND | 6.4 | ND | ND | ND | ND | ND |
| RSDs (%, n = 3) | 9.9 | - | 24.6 | - | - | - | - | - | |
| Recoveries (%, spiked with 10 ng L−1) | 90.8 | 81.9 | 83.4 | 83.1 | 73.4 | 76.8 | 81.2 | 81.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Liu, H.; Long, A.; Li, H.; Chen, C.; Feng, S.; Fan, J. Carbon Dot-Decorated Graphite Carbon Nitride Composites for Enhanced Solid-Phase Microextraction of Chlorobenzenes from Water. Nanomaterials 2022, 12, 335. https://doi.org/10.3390/nano12030335
Xu S, Liu H, Long A, Li H, Chen C, Feng S, Fan J. Carbon Dot-Decorated Graphite Carbon Nitride Composites for Enhanced Solid-Phase Microextraction of Chlorobenzenes from Water. Nanomaterials. 2022; 12(3):335. https://doi.org/10.3390/nano12030335
Chicago/Turabian StyleXu, Shengrui, Hailin Liu, Anying Long, Huimin Li, Changpo Chen, Suling Feng, and Jing Fan. 2022. "Carbon Dot-Decorated Graphite Carbon Nitride Composites for Enhanced Solid-Phase Microextraction of Chlorobenzenes from Water" Nanomaterials 12, no. 3: 335. https://doi.org/10.3390/nano12030335