Exploring Microbial-Based Green Nanobiotechnology for Wastewater Remediation: A Sustainable Strategy
Abstract
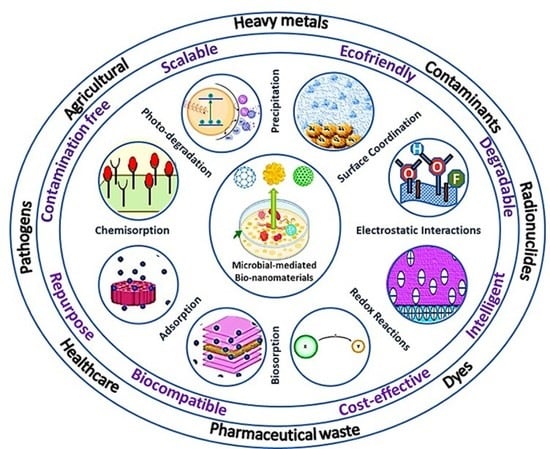
:1. Introduction
2. Nanotechnological Approaches for Wastewater Treatment
| Microorganisms | Biosynthesized NPs | Pollutant | Size Range (nm) | Characterization | Removal Efficiency | Mechanism of Pollutant Removal | References |
|---|---|---|---|---|---|---|---|
| Heavy metals | |||||||
| Manglicolous fungi | Iron oxide nanoparticles | Cr (VI) | 2–16 | UV-vis spectroscopy, FTIR spectroscopy, FESEM-EDX, TEM-EDX, XRD analyzer, VSM | >90% | Chemisorption | [32] |
| Aspergillus niger BSC-1 | Iron oxide NPs | 20–40 | UV-vis spectroscopy, ATR-FTIR spectroscopy, Raman spectroscopy, XRD, TEM, FESEM, zeta sizer, VSM | >99% | Adsorption and redox reactions | [33] | |
| Aspergillus terreus S1 | Magnesium Oxide NPs | 8.0–38.0 | UV-vis spectroscopy, FTIR spectroscopy, TEM, SEM-EDX, XRD, DLS | 97.5% | Precipitation and adsorption | [34] | |
| Marinobacter sp. MnI-79 | Manganese oxide NPs | Ag+ | - | XPS, surface analyzer | 95% | Electrostatic attraction and redox reactions | [35] |
| Streptomyces thermolineatus | Iron Oxide magnetic NPs | Cu | 22 | UV-vis spectroscopy, FTIR spectroscopy, SEM, TEM, XRD, thermogravimetric analysis, vibrating sample magnetometer, dynamic light scattering. | 85% | Interactions between electrostatic attraction, surface complexation, and coordination | [35] |
| Pseudomonas aeruginosa JP-11 | Cadmium Sulphide NPs | Cd(II) | 20–40 | UV-vis spectroscopy, FTIR spectroscopy, XRD, FESEM, TEM, AAS | 88.66% | Adsorption | [26] |
| Spirulina plantesis | Palladium NPs | Pb | 10–20 | UV-vis spectroscopy, XRD, FTIR, TEM | 90% | Adsorption | [36] |
| Aspergillus tubingenesis STSP 25 | Iron Oxide NPs | Pb(II) | 73.05 | UV-vis spectroscopy, Zeta analyser, DLS, FTIR, TEM-EDX, XRD, SQUID-VSM | 98% | Adsorption | [32] |
| Ni(II) | 96.45% | ||||||
| Cu(II) | 92.19% | ||||||
| Zn(II) | 93.99% | ||||||
| Dyes | |||||||
| Shewanellaoneidensis | Magnetite/reduced graphene oxide nanocomposite | Methylene blue | 11.0 | TEM, XRD, FTIR spectroscopy, XRP spectroscopy, vibrating sample magnetometry | 100% | Electrostatic attraction | [37] |
| Caldicellulosiruptorsaccharolyticus | Palladium NPs | Methyl orange and Diatrizoate | 10–20 | AAS, SEM, TEM-EDS | 100% | Reduction | [37] |
| Bacillus marisflavi TEZ7 [38] | Silver NPs | Direct Blue-1, Methyl Red & Reactive Black 5 | 11.20–39 | FTIR Spectroscopy, XRD, TEM, SEM, EDS | 54.14–96.92% | Photocatalytic degradation | [38] |
| Bacillus paralicheniformis, Bacillus pumilus, Sphingomonaspaucimobilis [39] | Silver NPs | Malachite green | 4–20 | XRD, TEM, FTIR spectroscopy | >90% | Adsorption | [39] |
| Spirulina plantensisn | 17.9 | UV-vis spectroscopy, XRD, TEM, FTIR spectroscopy | 88% | Biosorption | |||
| Anabaena variabilis [39] | 26.4 | 81% | |||||
| Pseudochrobactrum sp. C5 | Zinc Oxide NPs | Methanol blue and reactive black 5 | 90–110 | FTIR spectroscopy, XRD, FESEM | >90% | Catalytic degradation | [39] |
| Pharmaceutical and Hospital wastewater contaminants | |||||||
| Shewanellaoneiedensis MR-1 | Bio-Palladium NPs doped with Au(0) | Diclofenac | - | - | 43.8 ±5.5% | Catalytic degradation | [40] |
| Pseudonomas putida | Manganeese Oxide NPs | Estrone and 7α-ethinylestradiol | - | TEM-EDS, HPLC-MSMS | 100% | Absorption and oxidation | [41] |
| Desulfovibrio vulgaris | Platinum NPs | 17-β estradiol | - | TEM | 94% | Catalytic reduction | [39] |
| Sulfamethoxazole | 85% | ||||||
| Escherichia coli | Biogenic Palladium NPs | Ciprofloxacin | 10–30 | SEM, EDXA, XPS | 87.70% | Reductive degradation | [38] |
2.1. Removal and Recovery of Heavy Metals
2.2. Removal of Dyes
2.3. Removal of Pharmaceutical and Hospital Wastewater Pollutants
3. Bioaugmentation and Immobilization of Microbial Cells with Nanomaterials for Enhanced Wastewater Treatment
4. Integrated Microbial and Nanotechnological Approaches for Wastewater Treatment
4.1. Nanoparticle Integrated Microbial Reactor for the Removal of Pollutants from Wastewater
4.2. Microbial Nanofibrous Webs Filters (NFW) for Wastewater Treatment
4.3. Synergistic Combined Effect of Nanocomposites and Microbial Fuel Cells in Wastewater Treatment
4.4. Green Microbial Nanotechnology for Wastewater Treatment
5. Advancements and Future Approaches
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAS | Atomic Absorption Spectroscopy |
| AgNPs | Silver NPs |
| AMBR | Algal Membrane Bioreactor |
| CA-NFW | Cellulose Acetate Nanofibrous Webs |
| CNT | Carbon Nanotube |
| CrNPs | Chromium NPs |
| ECNM | Electrospun Chitosan Nanofiber Mats |
| EDS | Energy Dispersive Spectroscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| HPLC | High-Performance Liquid Chromatography |
| MDNPs | Manganese Dioxide NPs |
| MFC | Microbial Fuel Cell |
| NT | Nanotechnology |
| NPs | Nanoparticals |
| NMs | Nanomaterials |
| PdNPs | Palladium NPs |
| IONPs | Iron Oxide NPs |
| PES | polyethersulfone |
| PSF | polysulfone |
| PVA | Polyvinyl Alcohol |
| PVDF | polyvinylidene fluoride |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| VSM | Vibrating Sample Magnetometer |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X Ray Diffraction |
| ZnONPs | Zinc Oxide NPs |
| ZVI | Zero valent iron |
References
- Yadav, V.K.; Gupta, N.; Kumar, P.; Dashti, M.G.; Tirth, V.; Khan, S.H.; Yadav, K.K.; Islam, S.; Choudhary, N.; Algahtani, A.; et al. Recent Advances in Synthesis and Degradation of Lignin and Lignin Nanoparticles and Their Emerging Applications in Nanotechnology. Materials 2022, 15, 953. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Patel, A.; Pardhi, V.; Flora, S. Nanotechnology in wastewater management: A new paradigm towards wastewater treatment. Molecules 2021, 26, 1797. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, K.; Błaszczyk, M.; Sierota, Z.; Tkaczyk, M.; Oszako, T. Slow sand filtration for elimination of phytopathogens in water used in forest nurseries. Scand. J. For. Res. 2015, 30, 664–677. [Google Scholar] [CrossRef]
- Landowski, M.; Imielinska, K. Degradation of GFRP Marine Laminates with Nano Particle Modified Coatings. Adv. Mater. Sci. 2013, 13, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.A.; Sher, F.; Hameed, M.; Bashir, O.; Kumar, R.; Vo, D.-V.N.; Ahmad, P.; Lima, E.C. Sustainable nanotechnology based wastewater treatment strategies: Achievements, challenges and future perspectives. Chemosphere 2022, 288, 132606. [Google Scholar] [CrossRef]
- Ma, W.; Cao, W.; Lu, T.; Xiong, R.; Huang, C. Multifunctional nanofibrous membrane fabrication by a sacrifice template strategy for efficient emulsion oily wastewater separation and water purification. J. Environ. Chem. Eng. 2022, 10, 108908. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, Z.; Lu, T.; Xiong, R.; Huang, C. Lightweight, elastic and superhydrophobic multifunctional nanofibrous aerogel for self-cleaning, oil/water separation and pressure sensing. Chem. Eng. J. 2022, 430, 132989. [Google Scholar] [CrossRef]
- Ma, W.; Ding, Y.; Li, Y.; Gao, S.; Jiang, Z.; Cui, J.; Huang, C.; Fu, G. Durable, self-healing superhydrophobic nanofibrous membrane with self-cleaning ability for highly-efficient oily wastewater purification. J. Membr. Sci. 2021, 634, 119402. [Google Scholar] [CrossRef]
- Cao, W.; Ma, W.; Lu, T.; Jiang, Z.; Xiong, R.; Huang, C. Multifunctional nanofibrous membranes with sunlight-driven self-cleaning performance for complex oily wastewater remediation. J. Colloid Interface Sci. 2022, 608, 164–174. [Google Scholar] [CrossRef]
- Mandeep, D.; Shukla, P. Microbial nanotechnology for bioremediation of industrial wastewater. Front. Microbiol. 2020, 11, 590631. [Google Scholar] [CrossRef]
- Bera, S.P.; Godhaniya, M.; Kothari, C. Emerging and advanced membrane technology for wastewater treatment: A review. J. Basic Microbiol. 2022, 62, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Chabalala, M.B.; Gumbi, N.N.; Mamba, B.B.; Al-Abri, M.Z.; Nxumalo, E.N. Photocatalytic Nanofiber Membranes for the Degradation of Micropollutants and Their Antimicrobial Activity: Recent Advances and Future Prospects. Membranes 2021, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Duo, J.; Li, W.; Wang, Y.; Wang, S.; Wufuer, R.; Pan, X. Photothermal Catalytic Degradation of Lomefloxacin with Nano Au/TiO2. Water 2022, 14, 339. [Google Scholar] [CrossRef]
- Sikiru, S.; Abiodun, O.A.; Sanusi, Y.K.; Sikiru, Y.A.; Soleimani, H.; Yekeen, N.; Haslija, A.A. A comprehensive review on nanotechnology application in wastewater treatment a case study of metal-based using green synthesis. J. Environ. Chem. Eng. 2022, 10, 108065. [Google Scholar] [CrossRef]
- Kakorina, O.A.; Zaporotskova, I.V.; Kakorin, I.A. Detection of impurities in water by using nanostructures. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2022; Volume 2466. [Google Scholar]
- Kaminski, A.; Edwards, C.; Chrapusta-Srebrny, E.; Lawton, L.A. Anatoxin-a degradation by using titanium dioxide. Sci. Total Environ. 2021, 756, 143590. [Google Scholar] [CrossRef]
- Razack, S.A.; Duraiarasan, S. A way to create a sustainable environment: Green nanotechnology–with an emphasis on noble metals. In The ELSI Handbook of Nanotechnology: Risk, Safety, ELSI and Commercialization; Hussain, C.M., Ed.; Wiley-Scrivener: Austin, TX, USA, 2020; pp. 359–425. [Google Scholar]
- Khan, S. Green Materials for Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Nahak, B.K.; Preetam, S.; Sharma, D.; Shukla, S.; Syväjärvi, M.; Toncu, D.-C.; Tiwari, A. Advancements in net-zero pertinency of lignocellulosic biomass for climate neutral energy production. Renew. Sustain. Energy Rev. 2022, 161, 112393. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Roy, D.; Yadav, A.K.; Bharagava, R.N. Green synthesis of nanoparticles and their applications in water and wastewater treatment, in Bioremediation of industrial waste for environmental safety. In Bioremediation of Industrial Waste for Environmental Safety; SaxenaRam, G., Bharagava, N., Eds.; Springer: Singapore, 2020; pp. 349–379. [Google Scholar]
- Sivaraj, R.; Salam, H.A.; Rajiv, P.; Rajendran, V. Green nanotechnology: The solution to sustainable development of environment. In Environmental Sustainability; Springer: Berlin/Heidelberg, Germany, 2015; pp. 311–324. [Google Scholar]
- Nahak, B.K.; Mishra, A.; Preetam, S.; Tiwari, A. Advances in Organ-on-a-Chip Materials and Devices. ACS Appl. Bio Mater. 2022, 5, 3576–3607. [Google Scholar] [CrossRef]
- Preetam, S.; Nahak, B.K.; Patra, S.; Toncu, D.C.; Park, S.; Syväjärvi, M.; Orive, G.; Tiwari, A. Emergence of microfluidics for next generation biomedical devices. Biosens. Bioelectron. X 2022, 10, 100106. [Google Scholar] [CrossRef]
- Basheer, A.A. New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 2018, 261, 583–593. [Google Scholar] [CrossRef]
- Teye, G.K.; Darkwah, W.K.; Jingyu, H.; Ke, L.; Li, Y. Photodegradation of pharmaceutical and personal care products (PPCPs) and antibacterial activity in water by transition metals. Rev. Environ. Contam. Toxicol. 2020, 254, 131–162. [Google Scholar]
- Raja, R.K.; Hazir, S.; Balasubramani, G.; Sivaprakash, G.; Obeth, E.S.J.; Boobalan, T.; Pugazhendhi, A.; Raj, R.H.K.; Arun, A. Green nanotechnology for the environment. In Handbook of Microbial Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 461–478. [Google Scholar]
- Ciambelli, P.; Guardia, G.L.; Vitale, L. Nanotechnology for green materials and processes. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–116. [Google Scholar]
- Han, Y.; Lian, F.; Xiao, Z.; Gu, S.; Cao, X.; Wang, Z.; Xing, B. Potential toxicity of nanoplastics to fish and aquatic invertebrates: Current understanding, mechanistic interpretation, and meta-analysis. J. Hazard. Mater. 2021, 427, 127870. [Google Scholar] [CrossRef] [PubMed]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Yadav, V.; Ali, J.; Garg, M.C. Biosorption of methylene blue dye from textile-industry wastewater onto sugarcane bagasse: Response surface modeling, isotherms, kinetic and thermodynamic modeling. J. Hazard. Toxic Radioact. Waste 2021, 25, 04020067. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A comprehensive review on the chemical regeneration of biochar adsorbent for sustainable wastewater treatment. Npj Clean Water 2022, 5, 1–21. [Google Scholar] [CrossRef]
- Mahanty, S.; Bakshi, M.; Ghosh, S.; Chatterjee, S.; Bhattacharyya, S.; Das, P.; Das, S.; Chaudhuri, P. Green synthesis of iron oxide nanoparticles mediated by filamentous fungi isolated from Sundarban mangrove ecosystem, India. BioNanoScience 2019, 9, 637–651. [Google Scholar] [CrossRef]
- Das, P.; Barua, S.; Sarkar, S.; Chatterjee, S.K.; Mukherjee, S.; Goswami, L.; Das, S.; Bhattacharya, S.; Karak, N.; Bhattacharya, S.S. Mechanism of toxicity and transformation of silver nanoparticles: Inclusive assessment in earthworm-microbe-soil-plant system. Geoderma 2018, 314, 73–84. [Google Scholar] [CrossRef]
- Grandmontagne, D.; Navarro, D.; Neugnot-Roux, V.; Ladevèze, S.; Berrin, J.-G. The Secretomes of Aspergillus japonicus and Aspergillus terreus Supplement the Rovabio® Enzyme Cocktail for the Degradation of Soybean Meal for Animal Feed. J. Fungi 2021, 7, 278. [Google Scholar] [CrossRef]
- Gao, W.; Pei, A.; Dong, R.; Wang, J. Catalytic Iridium-Based Janus Micromotors Powered by Ultralow Levels of Chemical Fuels. J. Am. Chem. Soc. 2014, 136, 2276–2279. [Google Scholar] [CrossRef] [Green Version]
- Sayadi, M.H.; Salmani, N.; Heidari, A.; Rezaei, M.R. Bio-synthesis of palladium nanoparticle using Spirulina platensis alga extract and its application as adsorbent. Surf. Interfaces 2018, 10, 136–143. [Google Scholar] [CrossRef]
- Xiao, X.; Ma, X.-B.; Yuan, H.; Liu, P.-C.; Lei, Y.-B.; Xu, H.; Du, D.-L.; Sun, J.-F.; Feng, Y.-J. Photocatalytic properties of zinc sulfide nanocrystals biofabricated by metal-reducing bacterium Shewanella oneidensis MR-1. J. Hazard. Mater. 2015, 288, 134–139. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Ahmed, B.; Mehnaz, T.; Mehejabin, F.; Maliat, D.; Hoang, A.T.; Shafiullah, G. Nanomaterials as a sustainable choice for treating wastewater. Environ. Res. 2022, 214, 113807. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Mourato, C.; Sanches, S.; Noronha, J.P.; Crespo, M.B.; Pereira, I.A. Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds. Water Res. 2017, 108, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Luz, G.V.D.S.; Barros, K.V.G.; De Araújo, F.V.C.; Da Silva, G.B.; Da Silva, P.A.F.; Condori, R.C.I.; Brasil, L.M. Nanorobotics in drug delivery systems for treatment of cancer: A review. J. Mat. Sci. Eng. A 2016, 6, 167–180. [Google Scholar]
- Furgal, K.M.; Meyer, R.L.; Bester, K. Removing selected steroid hormones, biocides and pharmaceuticals from water by means of biogenic manganese oxide nanoparticles in situ at ppb levels. Chemosphere 2015, 136, 321–326. [Google Scholar] [CrossRef]
- Kishore, S.; Malik, S.; Shah, M.P.; Bora, J.; Chaudhary, V.; Kumar, L.; Sayyed, R.Z.; Ranjan, A. A comprehensive review on removal of pollutants from wastewater through microbial nanobiotechnology-based solutions. Biotech. Gen. Eng. Rev. 2022, 1–26. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Akhdhar, A.; Hamza, M.F. Recent advances in greenly synthesized nanoengineered materials for water/wastewater remediation: An overview. Nanotechnol. Environ. Eng. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Ghotekar, S.; Pansambal, S.; Lin, K.-Y.A.; Pore, D.; Oza, R. Recent Advances in Synthesis of CeVO4 Nanoparticles and Their Potential Scaffold for Photocatalytic Applications. Top. Catal. 2022, 1–15. [Google Scholar] [CrossRef]
- Lichtfouse, E. Environmental chemistry: Green Chemistry and Pollutants in Ecosystems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Bora, T.; Dutta, J. Applications of nanotechnology in wastewater Treatment—A review. J. Nanosci. Nanotechnol. 2014, 14, 613–626. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, R.; Kumar, H.; Li, H.; Zha, X.; Ouyang, F. Advanced applications and current status of green nanotechnology in the environmental industry. In Green Functionalized Nanomaterials for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 303–340. [Google Scholar]
- More, N.; Verma, A.; Wickramasinghe, D.; Gautam, R.; Navaratna, D.; Jonnada, A.V.P.R.; Bharagava, R.N. Applications of Nanoparticles for Microbial Contaminants and Pathogens Removal from Wastewater. In Nano-Biotechnology for Waste Water Treatment: Theory and Practices; Rai, J.P.N., Saraswat, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 213–226. [Google Scholar]
- Yadav, V.K.; Khan, S.H.; Malik, P.; Thappa, A.; Suriyaprabha, R.; Ravi, R.K.; Choudhary, N.; Kalasariya, H.; Gnanamoorthy, G. Microbial Synthesis of Nanoparticles and Their Applications for Wastewater Treatment. In Microbial Biotechnology: Basic Research and Applications; Singh, J., Vyas, A., Wang, S., Prasad, R., Eds.; Springer: Singapore, Singapore, 2020; pp. 147–187. [Google Scholar]
- Sharma, U.; Sharma, J.G. Nanotechnology for the bioremediation of heavy metals and metalloids. J. Appl. Biol. Biotechnol. 2022, 10, 34–44. [Google Scholar] [CrossRef]
- Bognár, S.; Putnik, P.; Šojić Merkulov, D. Sustainable green nanotechnologies for innovative purifications of water: Synthesis of the nanoparticles from renewable sources. Nanomaterials 2022, 12, 263. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Naz, I.; Khan, Z.M.; Sultan, M.; Islam, T.; Abbasi, I.A. Phytogenic magnetic nanoparticles for wastewater treatment: A review. RSC Adv. 2017, 7, 40158–40178. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.V.; Bhat, R.A.; Dervash, M.A.; Qadri, H.; Mehmood, M.A.; Dar, G.H.; Hameed, M.; Rashid, N. Wonders of nanotechnology for remediation of polluted aquatic environs. In Fresh Water Pollution Dynamics and Remediation; Springer: New York, NY, USA, 2020; pp. 319–339. [Google Scholar]
- Nath, D.; Banerjee, P. Green nanotechnology–a new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Kanchi, S.; Ahmed, S. Green Metal Nanoparticles: Synthesis, Characterization and their Applications; John Wiley & Sons: New York, NY, USA, 2018. [Google Scholar]
- Iavicoli, I.; Leso, V.; Ricciardi, W.; Hodson, L.L.; Hoover, M.D. Opportunities and challenges of nanotechnology in the green economy. Environ. Health 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Qahtani, K.M. Cadmium removal from aqueous solution by green synthesis zero valent silver nanoparticles with Benjamina leaves extract. Egypt. J. Aquat. Res. 2017, 43, 269–274. [Google Scholar] [CrossRef]
- Mahanty, S.; Tudu, P.; Ghosh, S.; Chatterjee, S.; Das, P.; Bhattacharyya, S.; Das, S.; Acharya, K.; Chaudhuri, P. Chemometric study on the biochemical marker of the manglicolous fungi to illustrate its potentiality as a bio indicator for heavy metal pollution in Indian Sundarbans. Mar. Pollut. Bull. 2021, 173, 113017. [Google Scholar] [CrossRef]
- Lim, C.K.; Bay, H.H.; Aris, A.; Majid, Z.A.; Ibrahim, Z. Biosorption and biodegradation of Acid Orange 7 by Enterococcus faecalis strain ZL: Optimization by response surface methodological approach. Environ. Sci. Pollut. Res. 2013, 20, 5056–5066. [Google Scholar] [CrossRef]
- Dell’Anno, F.; Rastelli, E.; Buschi, E.; Barone, G.; Beolchini, F.; Dell’Anno, A. Fungi Can Be More Effective than Bacteria for the Bioremediation of Marine Sediments Highly Contaminated with Heavy Metals. Microorganisms 2022, 10, 993. [Google Scholar] [CrossRef]
- Wong, S.; Karn, B. Ensuring sustainability with green nanotechnology. Nanotechnology 2012, 23, 290201. [Google Scholar] [CrossRef] [Green Version]
- Makarov, V.V.; Love, A.J.; Sinitsyana, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. ActaNaturae (Англoязычная Версия) 2014, 6, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.Z.N.; Salleh, W.N.W.; Ismail, A.F.; Yusof, N.; Yusop, M.Z.M.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef] [PubMed]
- Aghalari, Z.; Dahms, H.-U.; Sillanpää, M.; Sosa-Hernandez, J.E.; Parra-Saldívar, R. Effectiveness of wastewater treatment systems in removing microbial agents: A systematic review. Glob. Health 2020, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Gupta, R.; Agarwal, N. Advances in synthesis and applications of microalgal nanoparticles for wastewater treatment. J. Nanotechnol. 2019, 1–9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Abdelbasir, S.M.; Shalan, A.E. An overview of nanomaterials for industrial wastewater treatment. Korean J. Chem. Eng. 2019, 36, 1209–1225. [Google Scholar] [CrossRef]
- Arakaki, A.; Nakazawa, H.; Nemoto, M.; Mori, T.; Matsunaga, T. Formation of magnetite by bacteria and its application. J. R. Soc. Interface 2008, 5, 977–999. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.T.; Bazylinski, D.A. Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 497–526. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Zhang, S.; Chen, P.; Liu, H.; Yin, H.; Li, H. Magnetotactic bacteria, magnetosomes and their application. Microbiol. Res. 2012, 167, 507–519. [Google Scholar] [CrossRef]
- Guieysse, B.; Borde, X.; Muñoz, R.; Hatti-Kaul, R.; Nugier-Chauvin, C.; Patin, H.; Mattiasson, B. Influence of the initial composition of algal-bacterial microcosms on the degradation of salicylate in a fed-batch culture. Biotechnol. Lett. 2002, 24, 531–538. [Google Scholar] [CrossRef]
- Plata-Díaz, A.M.; Zafra-Gómez, J.L.; Pérez-López, G.; López-Hernández, A.M. Alternative management structures for municipal waste collection services: The influence of economic and political factors. Waste Manag. 2014, 34, 1967–1976. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Ergas, S.J.; van der Steen, P. A novel shortcut nitrogen removal process using an algal-bacterial consortium in a photo-sequencing batch reactor (PSBR). Water Res. 2015, 87, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, Y.; Yang, Y.; Zhou, D. The role of starvation in biomass harvesting and lipid accumulation: Co-culture of microalgae-bacteria in synthetic wastewater. Environ. Prog. Sustain. Energy 2016, 35, 103–109. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, J.; Yang, C.-W.; Wan, Y.; Tang, H.-L.; Aljarb, A.A.; Chen, C.; Fu, J.-H.; Wei, X.; Huang, K.-W.; et al. Mixed-dimensional MXene-hydrogel heterostructures for electronic skin sensors with ultrabroad working range. Sci. Adv. 2020, 6, eabb5367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.; Xiao, G.; Tang, Z.P.; Wang, M.Y. Simultaneous photocatalytic and microbial degradation of dye-containing wastewater by a novel g-C3N4-P25/photosynthetic bacteria composite. PLoS ONE 2017, 12, e0172747. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.; Noman, M.; Shahid, M.; Niazi, M.B.K.; Hussain, S.; Manzoor, N.; Wang, X.; Li, B. Green synthesis of silver nanoparticles transformed synthetic textile dye into less toxic intermediate molecules through LC-MS analysis and treated the actual wastewater. Environ. Res. 2020, 191, 110142. [Google Scholar] [CrossRef]
- AlMasoud, N.; Alhaik, H.; Almutairi, M.; Houjak, A.; Hazazi, K.; Alhayek, F.; Aljanoubi, S.; Alkhaibari, A.; Alghamdi, A.; Soliman, D.A.; et al. Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity. Green Process. Synth. 2021, 10, 518–528. [Google Scholar] [CrossRef]
- Azeez, L.; Adejumo, A.L.; Lateef, A.; Adebisi, S.A.; Adetoro, R.O.; Adewuyi, S.O.; Tijani, K.O.; Olaoye, S. Zero-valent silver nanoparticles attenuate Cd and Pb toxicities on Moringa oleifera via immobilization and induction of phytochemicals. Plant Physiol. Biochem. 2019, 139, 283–292. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Atar, N.; Eren, T.; Yola, M.L.; Wang, S. A sensitive molecular imprinted surface plasmon resonance nanosensor for selective determination of trace triclosan in wastewater. Sens. Actuators B Chem. 2015, 216, 638–644. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Kumar, L.; Mukerjee, N.; Anand, U.; Dhasmana, A.; Preetam, S.; Bhaumik, S.; Sihi, S.; Pal, S.; Khare, T.; et al. The emergence of metal oxide nanoparticles (NPs) as a phytomedicine: A two-facet role in plant growth, nano-toxicity and anti-phyto-microbial activity. Biomed. Pharmacother. 2022, 155, 113658. [Google Scholar] [CrossRef]
- Aqel, A.; Abou El-Nour, K.M.M.; Ammar, R.A.A.; Al-Warthan, A. Carbon nanotubes, science and technology part (I) structure, synthesis and characterisation. Arab. J. Chem. 2012, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- De Gusseme, B.; Hennebel, T.; Christiaens, E.; Saveyn, H.; Verbeken, K.; Fitts, J.P.; Boon, N.; Verstraete, W. Virus disinfection in water by biogenic silver immobilized in polyvinylidene fluoride membranes. Water Res. 2011, 45, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.L.A.; Adolphsen, C.; Akay, A.N.; Aksakal, H.; Albacete, J.L.; Alekhin, S.; Allport, P.; Andreev, V.; Appleby, R.B.; Arikan, E.; et al. A large hadron electron collider at CERN report on the physics and design concepts for machine and detector. J. Phys. G Nucl. Part. Phys. 2012, 39, 075001. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Andwari, A.M.; Wahid, M.A.; Bagheri, G. A review on green energy potentials in Iran. Renew. Sustain. Energy Rev. 2013, 27, 533–545. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Hou, R.; Chen, X.; Gao, Y.; Zhu, H.; Li, S.; Li, H.; Huo, Y. Synergistic Photocatalysis-Catalytic Peroxidation of BiOBr/H2O2 System for 4-Chlorophenol Degradation. ChemistrySelect 2016, 1, 1000–1003. [Google Scholar] [CrossRef]
- Alanazi, H.S.; Alotaibi, H.; Al-Shehri, H.S.; Alharthi, F.A. Green Synthesis of NiO Nanoparticle Using Ziziphus Spina-Christi Leaves Extract for Photocatalytic Methylene Blue Dye Degradation. Sci. Adv. Mater. 2021, 13, 1756–1763. [Google Scholar] [CrossRef]
- Arora, B.; Attri, P. Carbon nanotubes (CNTs): A potential nanomaterial for water purification. J. Compos. Sci. 2020, 4, 135. [Google Scholar] [CrossRef]
- Martinelli, L.; Nikel, P.I. Breaking the state-of-the-art in the chemical industry with new-to-Nature products via synthetic microbiology. Microb. Biotechnol. 2019, 12, 187. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, M.K.; Mahmoud, A.S.; SaryEl-deen, R.A.; Peters, R.W. Application of entrapped nano zero valent iron into cellulose acetate membranes for domestic wastewater treatment. In Proceedings of the Environmental Aspects, Applications and Implications of Nanomaterials and Nanotechnology 2017–Topical Conference at the 2017 AIChE Annual Meeting, Minneapolis, MN, USA, 1 November 2017. [Google Scholar]
- Bhatt, P.; Pandey, S.C.; Joshi, S.; Chaudhary, P.; Pathak, V.M.; Huang, Y.; Wu, X.; Zhou, Z.; Chen, S. Nanobioremediation: A sustainable approach for the removal of toxic pollutants from the environment. J. Hazard. Mater. 2022, 427, 128033. [Google Scholar] [CrossRef]
- Medina-Cruz, D.; Mostafavi, E.; Vernet-Crua, A.; Cheng, J.; Shah, V.; Cholula-Diaz, J.L.; Guisbiers, G.; Tao, J.; García-Martín, J.M.; Webster, T.J. Green nanotechnology-based drug delivery systems for osteogenic disorders. Expert Opin. Drug Deliv. 2020, 17, 341–356. [Google Scholar] [CrossRef]
- Preetam, S.; Dash, L.; Sarangi, S.S.; Sahoo, M.M.; Pradhan, A.K. Application of Nanobiosensor in Health Care Sector. In Bio-Nano Interface; Springer: Berlin/Heidelberg, Germany, 2022; pp. 251–270. [Google Scholar]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.M.O.; Bin-Meferij, M.M. Cyanobacteria–a promising platform in green nanotechnology: A review on nanoparticles fabrication and their prospective applications. Int. J. Nanomed. 2020, 15, 6033. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, A.P.; Varbanov, P.S.; Vocciante, M.; Fabiano, B. Bismuth oxide-related photocatalysts in green nanotechnology: A critical analysis. Front. Chem. Sci. Eng. 2018, 12, 878–892. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Multi-walled carbon nanotubes (MWNTs)/polysulfone (PSU) mixed matrix hollow fiber membranes for enhanced water treatment. J. Membr. Sci. 2013, 437, 237–248. [Google Scholar] [CrossRef]
- Verma, A.; Gautam, S.P.; Bansal, K.K.; Prabhakar, N.; Rosenholm, J.M. Green nanotechnology: Advancement in phytoformulation research. Medicines 2019, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.-H.; Chen, Y.-D.; Qu, W.-Y.; Liu, F.-Y.; Wang, Y. Algal culture and biofuel production using wastewater. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–198. [Google Scholar]
- Zhou, Y.; Schideman, L.; Yu, G.; Zhang, Y. A synergistic combination of algal wastewater treatment and hydrothermal biofuel production maximized by nutrient and carbon recycling. Energy Environ. Sci. 2013, 6, 3765–3779. [Google Scholar] [CrossRef]
- Gao, Z.; Raza, R.; Zhu, B.; Mao, Z.Q.; Wang, C.; Liu, Z.X. Preparation and characterization of Sm0.2Ce0.8O1.9/Na2CO3 nanocomposite electrolyte for low-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2011, 36, 3984–3988. [Google Scholar] [CrossRef]
- Neupane, G.P.; Ma, W.; Yildirim, T.; Tang, Y.; Zhang, L.; Lu, Y. 2D organic semiconductors, the future of green nanotechnology. Nano Mater. Sci. 2019, 1, 246–259. [Google Scholar] [CrossRef]
- Dutta, D.; Das, B.M. Scope of green nanotechnology towards amalgamation of green chemistry for cleaner environment: A review on synthesis and applications of green nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100418. [Google Scholar] [CrossRef]












| Algal + Bacteria Strain | Type of Wastewater | Pollutant Removal | References | |||
|---|---|---|---|---|---|---|
| COD (%) | Nitrogen (%) | Phosphorus (%) | ||||
| Chlorella sp. | + activated sludge | Synthetic wastewater | 87 | 99 | 83 | [72] |
| Algal | + bacteria | Synthetic wastewater | 96 | 46 | 45 | [72] |
| Chlorella vulgaris | + activated sludge | Municipal wastewater | 55 | 95 | 100 | [73] |
| Scenedesmus sp. | + Bacteria group | Municipal wastewater | 92 | 95 | 98 | [73] |
| Selenastrum sp. | + bacteria | Composting leachate liquids | NA | >90 | 92 | [73] |
| Chlorella vulgaris | + Pseudomonas putida | Synthetic wastewater | 85 | 85 | 66 | [74] |
| Chlorella vulgaris | + Enterobacter asburiae | Synthetic wastewater | 95 | 100 | 96 | [74] |
| + Klebsiella sp. | Synthetic wastewater | 85 | 97 | 95 | ||
| + Raoultella ornithinolytica | Synthetic wastewater | 89 | 100 | 95 | ||
| + Pseudomonas putida | Municipal wastewater | 97 | 100 | 100 | ||
| + Bacillus licheniformis | Synthetic wastewater | 86 | 88 | 80 | ||
| Chlorella vulgaris | + activated sludge | Synthetic wastewater | 83 | 89.4 | 91.4 | [75] |
| Consortium of algae, consisting primarily of Chlorella, Chlamydomonas, and Stichococcus | + bacteria | Anaerobically digested swine manure | NA | NA | 90 | [76] |
| Microcystis aeruginosa | + Bacillus licheniformis | Synthetic wastewater | 65 | 21.56 | 70 | [19] |
| Pollutants | Matrix Composition | Microbial Strain | Removal Efficiency |
|---|---|---|---|
| Cu (II) Peng et al., 2010 [85] | Chitosan-magnetic NPs | Saccharomyces cerevisiae | 96.8% |
| Diatrizoate [86] | PdNPs | Shewanellaoneidensis MR-1 | 77% |
| Nitrate [87] | Chitosan nanofibers | Chlorella vulgaris | 87% |
| Graphene nanosheets | 9 mg L−1 day−1 | ||
| Pb(II) [88] | Calcium alginate-IONPs | Phanerochaetechrysosporium | 96.03% |
| Atrazine [88] | CNTs | Arthrobacter sp. | 100% |
| Chlorophenol [89] | Magnetic NPs | Rhodococcus rhodochrous | 90% |
| Decolourization of industrial effluents [90] | Silica NPs | Actinomycetes | 80% |
| Bacterial Type | Nanoparticles | Microbial Strain | Pollutants | Removal Efficiency |
|---|---|---|---|---|
| Gram-negative facultative gamma proteobacterium [85] | PdNPs | Shewanellaoneidensis MR-1 | Trichloroethylene | 100% 98% |
| Membranes integrating PdNPs | Shewanellaoneidensis MR-1 | Diatrizoate | 77% | |
| Bio-Pd beads bio-Pd polyurethane cubes | Shewanellaoneidensis | Trichloroethylene | 98% | |
| Gram-negative bacteria [85] | bio-Pd in MBR | Citrobacter braakii (ATCC 6750) | Diatrizoate | 100% |
| Gram-negative, rod-shaped, saprotrophic soil bacterium [92] | MnONPs in MBR | Pseudomonas putida | Ibuprofen Naproxen Diuron Codeine N-acetylsulfamethoxazole Chlorophene Diclofenac Mecoprop | >95% >95% >94% >93% 92% >89% 86% 81% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, S.; Dhasmana, A.; Preetam, S.; Mishra, Y.K.; Chaudhary, V.; Bera, S.P.; Ranjan, A.; Bora, J.; Kaushik, A.; Minkina, T.; et al. Exploring Microbial-Based Green Nanobiotechnology for Wastewater Remediation: A Sustainable Strategy. Nanomaterials 2022, 12, 4187. https://doi.org/10.3390/nano12234187
Malik S, Dhasmana A, Preetam S, Mishra YK, Chaudhary V, Bera SP, Ranjan A, Bora J, Kaushik A, Minkina T, et al. Exploring Microbial-Based Green Nanobiotechnology for Wastewater Remediation: A Sustainable Strategy. Nanomaterials. 2022; 12(23):4187. https://doi.org/10.3390/nano12234187
Chicago/Turabian StyleMalik, Sumira, Archna Dhasmana, Subham Preetam, Yogendra Kumar Mishra, Vishal Chaudhary, Sweta Parmita Bera, Anuj Ranjan, Jutishna Bora, Ajeet Kaushik, Tatiana Minkina, and et al. 2022. "Exploring Microbial-Based Green Nanobiotechnology for Wastewater Remediation: A Sustainable Strategy" Nanomaterials 12, no. 23: 4187. https://doi.org/10.3390/nano12234187