Rapid and Effective Way to Synthesize Highly Crystalline Nanosized SAPO-34 Particles
Abstract
:1. Introduction
2. Experimental Section
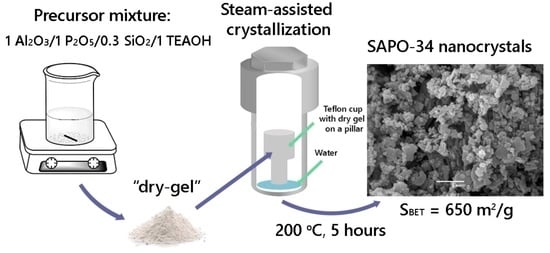
2.1. Materials and SAPO-34 Synthesis Procedure
2.2. Procedure for Obtaining SAPO-34-Covered Aluminum Heat Exchanger
2.3. Characterization
3. Results and Discussion
3.1. SAPO-34 Properties
3.2. Primary Experiments on Obtaining Aluminum Heat Exchanger Covered by SAPO-34
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AdHEX | Adsorber heat exchanger |
| AEI | Aluminum phosphate—Eighteen (International Zeolite Association code) |
| AIP | Aluminum isopropoxide |
| BET | Brunauer–Emmett–Teller theory |
| BJH | Barrett–Joyner–Halenda |
| CHA | Chabazite |
| DEA | Diethylamine |
| DGC | Dry gel conversion |
| HEX | Heat exchanger |
| HF | Hierarchy factor |
| MOR | Morpholine |
| MTO | Methanol-to-olefins |
| NH3-TPD | ammonia temperature-programmed desorption |
| SAC | Steam-assisted crystallization |
| SAPO | Silicoaluminophosphate(s) |
| SDA | Structure-directing agent |
| SEM | Scanning electron microscopy |
| TEA | Triethylamine |
| TEAOH | Tetraethylammonium hydroxide |
| VPT | Vapor-phase transport |
| XRD | X-ray diffraction |
References
- Flanigen, E.M.; Lok, B.M.; Patton, R.L.; Wilson, S.T. Aluminophosphate Molecular Sieves and the Periodic Table. Stud. Surf. Sci. Catal. 1986, 28, 103–112. [Google Scholar]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-53064-6. [Google Scholar]
- Jabłońska, M. Recent Progress in the Selective Catalytic Reduction of NO with NH3 on Cu-SAPO-34 Catalysts. Mol. Catal. 2022, 518, 112111–112133. [Google Scholar] [CrossRef]
- Shamanaeva, I.A.; Yu, Z.; Utemov, A.V.; Wu, W.; Sladkovskiy, D.A.; Parkhomchuk, E.V. Role of Texture and Acidity of SAPO-34 in Methanol to Olefins Conversion. Pet. Chem. 2020, 60, 471–478. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Guo, S.; Qin, Z.; Dong, M.; Wang, J.; Fan, W. Effective Conversion of CO2 into Light Olefins along with Generation of Low Amounts of CO. J. Catal. 2022, 413, 923–933. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A Short Review of Recent Advances in CO2 Hydrogenation to Hydrocarbons over Heterogeneous Catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Proverbio, E.; Freni, A. SAPO-34 Based Zeolite Coatings for Adsorption Heat Pumps. Energy 2019, 187, 115981–115988. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, R.Z.; Ge, T.S.; Hu, L.M. Performance Study of SAPO-34 and FAPO-34 Desiccants for Desiccant Coated Heat Exchanger Systems. Energy 2015, 93, 88–94. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M. Nanosized and Hierarchical Zeolites: A Short Review. Chin. J. Catal. 2016, 37, 447–467. [Google Scholar] [CrossRef]
- Tosheva, L.; Valtchev, V.P. Nanozeolites: Synthesis, Crystallization Mechanism, and Applications. Chem. Mater. 2005, 17, 2494–2513. [Google Scholar] [CrossRef]
- Askari, S.; Siahmard, A.B.; Halladj, R.; Alipour, S.M. Different Techniques and Their Effective Parameters in Nano SAPO-34 Synthesis: A Review. Powder Technol. 2016, 301, 268–287. [Google Scholar] [CrossRef]
- Yu, W.; Wu, X.; Cheng, B.; Tao, T.; Min, X.; Mi, R.; Huang, Z.; Fang, M.; Liu, Y. Synthesis and Applications of SAPO-34 Molecular Sieves. Chem. Eur. J. 2022, 28, e202102787. [Google Scholar] [PubMed]
- Zhang, L.; Bates, J.; Chen, D.; Nie, H.Y.; Huang, Y. Investigations of Formation of Molecular Sieve SAPO-34. J. Phys. Chem. C 2011, 115, 22309–22319. [Google Scholar] [CrossRef]
- Rimaz, S.; Halladj, R.; Askari, S. Synthesis of Hierarchal SAPO-34 Nano Catalyst with Dry Gel Conversion Method in the Presence of Carbon Nanotubes as a Hard Template. J. Colloid Interface Sci. 2016, 464, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Askari, S.; Sedighi, Z.; Halladj, R. Rapid Synthesis of SAPO-34 Nanocatalyst by Dry Gel Conversion Method Templated with Morphline: Investigating the Effects of Experimental Parameters. Microporous Mesoporous Mater. 2014, 197, 229–236. [Google Scholar] [CrossRef]
- Rezaei, Y.; Halladj, R.; Askari, S.; Tarjomannejad, A.; Rezaei, T. High-Temperature Synthesis of SAPO-34 Molecular Sieve Using a Dry Gel Method. Particuology 2016, 27, 61–65. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y. Crystallization and Catalytic Properties of Molecular Sieve SAPO-34 by a Vapor-Phase Transport Method. J. Mater. Chem. A 2015, 3, 4522–4529. [Google Scholar] [CrossRef]
- Di, C.Y.; Li, X.F.; Wang, P.; Li, Z.H.; Fan, B.B.; Dou, T. Green and Efficient Dry Gel Conversion Synthesis of SAPO-34 Catalyst with Plate-like Morphology. Pet. Sci. 2017, 14, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.H.; Li, X.F.; Di, C.Y.; Dou, T.; Chen, S.L. A Green and Cost-Effective Synthesis of Hierarchical SAPO-34 through Dry Gel Conversion and Its Performance in a Methanol-to-Olefin Reaction. Ind. Eng. Chem. Res. 2021, 60, 15380–15390. [Google Scholar] [CrossRef]
- Hirota, Y.; Murata, K.; Tanaka, S.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Dry Gel Conversion Synthesis of SAPO-34 Nanocrystals. Mater. Chem. Phys. 2010, 123, 507–509. [Google Scholar] [CrossRef]
- Riyar, B.K.; Agarwal, V.K. Synthesis of SAPO-34 Using the Different Combinations of Four Templates by Dry Gel Conversion Method. Braz. J. Chem. Eng. 2021, 38, 887–900. [Google Scholar] [CrossRef]
- Peréz-Ramírez, J.; Verboekend, D.; Bonilla, A.; Abelló, S. Zeolite Catalysts with Tunable Hierarchy Factor by Pore-Growth Moderators. Adv. Funct. Mater. 2009, 19, 3972–3979. [Google Scholar] [CrossRef]
- Poulet, G.; Tuel, A.; Sautet, P. A Combined Experimental and Theoretical Evaluation of the Structure of Hydrated Microporous Aluminophosphate AlPO4-18. J. Phys. Chem. B 2005, 109, 22939–22946. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.L.; Jiang, Y.J.; Cao, Y.Q.; Chen, F.; Chang, W.K.; Gao, Y.L. Influence of Template Content on Selective Synthesis of SAPO-18, SAPO-18/34 Intergrowth and SAPO-34 Molecular Sieves Used for Methanol-to-Olefins Process. RSC Adv. 2016, 6, 104985–104994. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Li, Z.; Wang, Y.; Yu, J. Synthesis of SAPO-18/34 Intergrowth Zeolites and Their Enhanced Stability for Dimethyl Ether to Olefins. RSC Adv. 2017, 7, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Izadbakhsh, A.; Farhadi, F.; Khorasheh, F.; Sahebdelfar, S.; Asadi, M.; Feng, Y.Z. Effect of SAPO-34’s Composition on Its Physico-Chemical Properties and Deactivation in MTO Process. Appl. Catal. A Gen. 2009, 364, 48–56. [Google Scholar] [CrossRef]
- Buchholz, A.; Wang, W.; Xu, M.; Arnold, A.; Hunger, M. Thermal Stability and Dehydroxylation of Brønsted Acid Sites in Silicoaluminophosphates H-SAPO-11, H-SAPO-18, H-SAPO-31, and H-SAPO-34 Investigated by Multi-Nuclear Solid-State NMR Spectroscopy. Microporous Mesoporous Mater. 2002, 56, 267–278. [Google Scholar] [CrossRef]
- Danilina, N.; Krumeich, F.; Van Bokhoven, J.A. Hierarchical SAPO-5 Catalysts Active in Acid-Catalyzed Reactions. J. Catal. 2010, 272, 37–43. [Google Scholar] [CrossRef]
- Guo, L.; Bao, X.; Fan, Y.; Shi, G.; Liu, H.; Bai, D. Impact of Cationic Surfactant Chain Length during SAPO-11 Molecular Sieve Synthesis on Structure, Acidity, and n-Octane Isomerization to Di-Methyl Hexanes. J. Catal. 2012, 294, 161–170. [Google Scholar] [CrossRef]
- Li, X.; Rezaei, F.; Ludlow, D.K.; Rownaghi, A.A. Synthesis of SAPO-34@ZSM-5 and SAPO-34@Silicalite-1 Core-Shell Zeolite Composites for Ethanol Dehydration. Ind. Eng. Chem. Res. 2018, 57, 1446–1453. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore Size Determination in Modified Micro- and Mesoporous Materials. Pitfalls and Limitations in Gas Adsorption Data Analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A. Hierarchy Concepts: Classification and Preparation Strategies for Zeolite Containing Materials with Hierarchical Porosity. Chem. Soc. Rev. 2016, 45, 3353–3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastre, G.; Lewis, D.; Catlow, C. Mechanisms of silicon incorporation in aluminophosphate molecular sieves. J. Mol. Catal. A Chem. 1997, 119, 349–356. [Google Scholar] [CrossRef]
- Freni, A.; Bonaccorsi, L.; Calabrese, L.; Caprì, A.; Frazzica, A.; Sapienza, A. SAPO-34 coated adsorbent heat exchanger for adsorption chillers. Appl. Therm. Eng. 2015, 82, 1–7. [Google Scholar] [CrossRef]
- Freni, A.; Frazzica, A.; Dawoud, B.; Chmielewski, S.; Calabrese, L.; Bonaccorsi, L. Adsorbent coatings for heat pumping applications: Verification of hydrothermal and mechanical stabilities. Appl. Therm. Eng. 2013, 50, 1658–1663. [Google Scholar] [CrossRef]
- Lang, R.; Roth, M.; Stricker, M.; Westerfeld, T. Development of a modular zeolite-water heat pump. Heat Mass Transf. 1999, 35, 229–234. [Google Scholar] [CrossRef]
- Restuccia, G. Zeolite heat pump for domestic heating. Energy 1988, 13, 333–342. [Google Scholar] [CrossRef]
- Tatlıer, M.; Erdem-Şenatalar, A. Optimization of the cycle durations of adsorption heat pumps employing zeolite coatings synthesized on metal supports. Microporous Mesoporous Mater. 2000, 34, 23–30. [Google Scholar] [CrossRef]
- Feng, C.; E, J.; Han, W.; Deng, Y.; Zhang, B.; Zhao, X.; Han, D. Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: A review. Renew. Sustain. Energy Rev. 2021, 144, 110954–110981. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calabrese, L.; Freni, A.; Proverbio, E.; Restuccia, G. Zeolites direct synthesis on heat exchangers for adsorption heat pumps. Appl. Therm. Eng. 2013, 50, 1590–1595. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Calabrese, L.; Freni, A.; Proverbio, E. Hydrothermal and microwave synthesis of SAPO (CHA) zeolites on aluminium foams for heat pumping applications. Microporous Mesoporous Mater. 2013, 167, 30–37. [Google Scholar] [CrossRef]









| Sample | SBET, m2/g | Sexternal, m2/g | Vmicro, cm3/g | Vmeso, cm3/g | HF |
|---|---|---|---|---|---|
| SAPO-34–5h | 651 | 68 | 0.22 | 0.29 | 0.045 |
| SAPO-34–24h | 695 | 52 | 0.24 | 0.32 | 0.032 |
| SAPO-34–B–5h | 668 | 57 | 0.23 | 0.46 | 0.028 |
| Sample | n NH3 (161–202 °C), mmol/g | n NH3 (212–266 °C), mmol/g | n NH3 (451–454 °C), mmol/g | Total Amount, mmol/g |
|---|---|---|---|---|
| SAPO-34–5h | 0.29 | 0.64 | 0.44 | 1.37 |
| SAPO-34–24h | 0.27 | 0.77 | 0.37 | 1.41 |
| SAPO-34–B–5h | 0.60 | 0.24 | 0.33 | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamanaeva, I.; Strelova, S.; Solovyeva, M.; Grekova, A. Rapid and Effective Way to Synthesize Highly Crystalline Nanosized SAPO-34 Particles. Nanomaterials 2022, 12, 4086. https://doi.org/10.3390/nano12224086
Shamanaeva I, Strelova S, Solovyeva M, Grekova A. Rapid and Effective Way to Synthesize Highly Crystalline Nanosized SAPO-34 Particles. Nanomaterials. 2022; 12(22):4086. https://doi.org/10.3390/nano12224086
Chicago/Turabian StyleShamanaeva, Irina, Svetlana Strelova, Marina Solovyeva, and Aleksandra Grekova. 2022. "Rapid and Effective Way to Synthesize Highly Crystalline Nanosized SAPO-34 Particles" Nanomaterials 12, no. 22: 4086. https://doi.org/10.3390/nano12224086