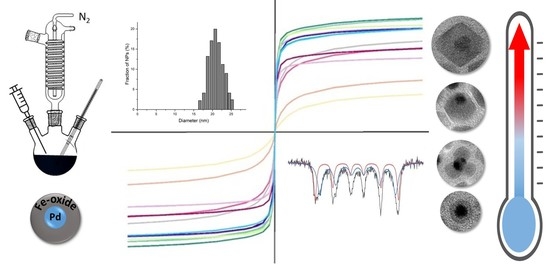
From Structure to Function: Understanding Synthetic Conditions in Relation to Magnetic Properties of Hybrid Pd/Fe-Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Pd/Fe-Oxide NPs via a One-Pot Synthesis Method
2.2.1. Flower-like Pd/Fe-Oxide NPs
2.2.2. Popcorn-like Pd/Fe-Oxide NPs
2.3. Preparation of Pd-Seeds
2.3.1. Preparation of Oleylamine Capped Pd NPs (Seeds)
2.3.2. Preparation of N-dodecyl Sulfide Capped Pd NPs (Seeds)
2.4. Preparation of Pd/Fe-Oxide NPs via a Seed-Mediated Method
2.5. Dispersion of Pd/Fe-Oxide NPs in Aqueous Media with DSPE-PEG2000-COOH
2.6. Characterization
3. Results and Discussion
3.1. Nanoparticles Synthesis
3.2. Tuning Parameters in the Seed-Mediated Growth Procedure
3.2.1. Effect of the [Fe]/[Pd] Ratio
3.2.2. Effect Pd-Seeds Coating Layer
3.2.3. Effect Heating Rate
3.2.4. Effect Surfactant to Iron Ratio
3.2.5. Effect Solvent/Temperature
3.3. Magnetic Properties of Pd/Fe-Oxide NPs as a Function of Their Morphology
3.4. Magnetic Hyperthermia and Relaxivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Gossuin, Y.; Gillis, P.; Hocq, A.; Vuong, Q.L.; Roch, A. Magnetic resonance relaxation properties of superparamagnetic particles. WIREs Nanomed. Nanobiotechnol. 2009, 1, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lamichhane, N.; Parul Sen, T.; Roy, I. Iron oxide nanoparticles conjugated with organic optical probes for in vivo diagnostic and therapeutic applications. Nanomedicine 2021, 16, 943–962. [Google Scholar] [CrossRef]
- Torres, M.d.R.R.; Tavare, R.; Paul, R.L.; Jauregui-Osoro, M.; Protti, A.; Glaria, A.; Varma, G.; Szanda, I.; Blower, P.J. Synthesis of 64Cu(II)-bis(dithiocarbamatebisphosphonate) and its conjugation with superparamagnetic iron oxide nanoparticles: In vivo evaluation as dual-modality PET-MRI agent. Angew. Chem. Int. Ed. Engl. 2011, 50, 5509–5513. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, L.M.; Alvarez, V.A. Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices. Bioengineering 2019, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Boros, E.; Bowen, A.M.; Josephson, L.; Vasdev, N.; Holland, J.P. Chelate-free metal ion binding and heat-induced radiolabeling of iron oxide nanoparticles. Chem. Sci. 2015, 6, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Ma, D. Chapter 1—Hybrid Nanoparticles: An Introduction. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 3–6. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Qi, H.; Yan, B.; Li, C.; Lu, W. Synthesis and characterization of water-soluble magnetite nanocrystals via one-step sol-gel pathway. Sci. China Phys. Mech. Astron. 2011, 54, 1239–1243. [Google Scholar] [CrossRef]
- Jarzyna, P.A.; Skajaa, T.; Gianella, A.; Cormode, D.P.; Samber, D.D.; Dickson, S.D.; Chen, W.; Griffioen, A.W.; Fayad, Z.A.; Mulder, W.J.M. Iron oxide core oil-in-water emulsions as a multifunctional nanoparticle platform for tumor targeting and imaging. Biomaterials 2009, 30, 6947–6954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotin, G.; Perton, F.; Blanco-Andujar, C.; Pichon, B.; Mertz, D.; Bégin-Colin, S. Chapter 2—Design of Anisotropic Iron-Oxide-Based Nanoparticles for Magnetic Hyperthermia. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Fratila, R.M., De La Fuente, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–60. [Google Scholar] [CrossRef]
- Chai, Y.; Feng, F.; Li, Q.; Yu, C.; Feng, X.; Lu, P.; Yu, X.; Ge, M.; Wang, X.; Yao, L. One-Pot Synthesis of High-Quality Bimagnetic Core/Shell Nanocrystals with Diverse Exchange Coupling. J. Am. Chem. Soc. 2019, 141, 3366–3370. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Gilroy, K.D.; Peng, H.-C.; Xia, X. Seed-Mediated Growth of Colloidal Metal Nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 60–95. [Google Scholar] [CrossRef]
- Tancredi, P.; da Costa, L.S.; Calderon, S.; Moscoso-Londoño, O.; Socolovsky, L.M.; Ferreira, P.J.; Muraca, D.; Zanchet, D.; Knobel, M. Exploring the synthesis conditions to control the morphology of gold-iron oxide heterostructures. Nano Res. 2019, 12, 1781–1788. [Google Scholar] [CrossRef]
- Carbone, L.; Cozzoli, P.D. Colloidal heterostructured nanocrystals: Synthesis and growth mechanisms. Nano Today 2010, 5, 449–493. [Google Scholar] [CrossRef]
- Fantechi, E.; Roca, A.G.; Sepúlveda, B.; Torruella, P.; Estradé, S.; Peiró, F.; Coy, E.; Jurga, S.; Bastús, N.G.; Nogués, J.; et al. Seeded Growth Synthesis of Au–Fe3O4 Heterostructured Nanocrystals: Rational Design and Mechanistic Insights. Chem. Mater. 2017, 29, 4022–4035. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Klajn, R.; Pinchuk, A.O.; Grzybowski, B.A. Synthesis, Shape Control, and Optical Properties of Hybrid Au/Fe3O4 “Nanoflowers”. Small 2008, 4, 1635–1639. [Google Scholar] [CrossRef]
- Jang, Y.; Chung, J.; Kim, S.; Jun, S.W.; Kim, B.H.; Lee, D.W.; Kim, B.M.; Hyeon, T. Simple synthesis of Pd–Fe3O4 heterodimer nanocrystals and their application as a magnetically recyclable catalyst for Suzuki cross-coupling reactions. Phys. Chem. Chem. Phys. 2011, 13, 2512–2516. [Google Scholar] [CrossRef]
- Lee, J.; Chung, J.; Byun, S.M.; Kim, B.M.; Lee, C. Direct catalytic C–H arylation of imidazo[1,2-a]pyridine with aryl bromides using magnetically recyclable Pd–Fe3O4 nanoparticles. Tetrahedron 2013, 69, 5660–5664. [Google Scholar] [CrossRef]
- Jang, S.; Hira, S.A.; Annas, D.; Song, S.; Yusuf, M.; Park, J.C.; Park, S.; Park, K.H. Recent Novel Hybrid Pd–Fe3O4 Nanoparticles as Catalysts for Various C–C Coupling Reactions. Processes 2019, 7, 422. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-S.; Byun, S.; Kwon, J.; Kim, B.M. Magnetic Pd–Fe3O4 Heterodimer Nanocrystals as Recoverable Catalysts for Ligand-Free Hiyama Cross-Coupling Reactions. Bull. Korean Chem. Soc. 2016, 37, 1992–1997. [Google Scholar] [CrossRef]
- Yu, Y.; Anderson, L.L.; Li, Z.; Mellenberg, D.E.; Nath, R.; Schell, M.C.; Waterman, F.M.; Wu, A.; Blasko, J.C. Permanent prostate seed implant brachytherapy: Report of the American Association of Physicists in Medicine Task Group No. 64. Med. Phys. 1999, 26, 2054–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, J.; Cantor, A.; Solc, Z.; Huff, W.; Chovnick, S.D.; Behar, R.J.; Perez, R.; Otheguy, J.; Rabinowitz, R. 103Pd brachytherapy versus radical prostatectomy in patients with clinically localized prostate cancer: A 12-year experience from a single group practice. Brachytherapy 2005, 4, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Kainz, K. Radiation Oncology Physics: A Handbook for Teachers and Students. Med. Phys. 2006, 33, 371–396. [Google Scholar] [CrossRef]
- Maier, A.; Djanashvili, K.; Denkova, A.G.; van Rhoon, G.C.; Pignol, J.-P.; van Oossanen, R. Synthesis of Hybride Palladium(103)/Iron Oxide Nanoparticles for Thermobrachy Therapy. Eur. Pat. Appl. 2021, 33, 165–169. [Google Scholar]
- Chen, S.; Si, R.; Taylor, E.; Janzen, J.; Chen, J. Synthesis of Pd/Fe3O4 Hybrid Nanocatalysts with Controllable Interface and Enhanced Catalytic Activities for CO Oxidation. J. Phys. Chem. C 2012, 116, 12969–12976. [Google Scholar] [CrossRef]
- Lin, F.-H.; Chen, W.; Liao, Y.-H.; Doong, R.-A.; Li, Y. Effective approach for the synthesis of monodisperse magnetic nanocrystals and M-Fe3O4 (M = Ag, Au, Pt, Pd) heterostructures. Nano Res. 2011, 4, 1223–1232. [Google Scholar] [CrossRef]
- Mazumder, V.; Sun, S. Oleylamine-Mediated Synthesis of Pd Nanoparticles for Catalytic Formic Acid Oxidation. J. Am. Chem. Soc. 2009, 131, 4588–4589. [Google Scholar] [CrossRef]
- Ganesan, M.; Freemantle, R.G.; Obare, S.O. Monodisperse Thioether-Stabilized Palladium Nanoparticles: Synthesis, Characterization, and Reactivity. Chem. Mater. 2007, 19, 3464–3471. [Google Scholar] [CrossRef]
- Liu, S.; Guo, S.; Sun, S.; You, X.-Z. Dumbbell-like Au-Fe3O4 nanoparticles: A new nanostructure for supercapacitors. Nanoscale 2015, 7, 4890–4893. [Google Scholar] [CrossRef]
- Jin, Y.; Jia, C.; Huang, S.-W.; O’Donnell, M.; Gao, X. Multifunctional nanoparticles as coupled contrast agents. Nat. Commun. 2010, 1, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klencsár, Z. Mössbauer spectrum analysis by Evolution Algorithm. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1997, 129, 527–533. [Google Scholar] [CrossRef]
- Wang, C.; Yin, H.; Dai, S.; Sun, S. A General Approach to Noble Metal−Metal Oxide Dumbbell Nanoparticles and Their Catalytic Application for CO Oxidation. Chem. Mater. 2010, 22, 3277–3282. [Google Scholar] [CrossRef]
- Lassenberger, A.; Grünewald, T.A.; van Oostrum, P.D.J.; Rennhofer, H.; Amenitsch, H.; Zirbs, R.; Lichtenegger, H.C.; Reimhult, E. Monodisperse Iron Oxide Nanoparticles by Thermal Decomposition: Elucidating Particle Formation by Second-Resolved in Situ Small-Angle X-ray Scattering. Chem. Mater. 2017, 29, 4511–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.-M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Maity, D.; Choo, S.-G.; Yi, J.; Ding, J.; Xue, J.M. Synthesis of magnetite nanoparticles via a solvent-free thermal decomposition route. J. Magn. Magn. Mater. 2009, 321, 1256–1259. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Xie, J.; Peng, S.; Brower, N.; Pourmand, N.; Wang, S.X.; Sun, S. One-pot synthesis of monodisperse iron oxide nanoparticles for potential biomedical applications. Pure Appl. Chem. 2006, 78, 1003–1014. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S. Dumbbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.-A.; et al. Magnetite-Gold nanohybrids as ideal all-in-one platforms for theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef] [Green Version]
- Han, D.H.; Wang, J.P.; Luo, H.L. Crystallite size effect on saturation magnetization of fine ferrimagnetic particles. J. Magn. Magn. Mater. 1994, 136, 176–182. [Google Scholar] [CrossRef]
- Vargas, J.M.; Lawton, J.; Vargas, N.M.; Schuller, I.K.; Sowko, N.J.; Huang, M.-X.; Zhang, M. Temperature trends and correlation between SQUID superparamagnetic relaxometry and dc-magnetization on model iron-oxide nanoparticles. J. Appl. Phys. 2020, 127, 044304. [Google Scholar] [CrossRef]
- Skumryev, V.; Stoyanov, S.; Zhang, Y.; Hadjipanayis, G.; Givord, D.; Nogués, J. Beating the superparamagnetic limit with exchange bias. Nature 2003, 423, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [Green Version]
- Saragi, T.; Sinaga, H.D.; Rahmi, F.; Pramesti, G.A.; Sugiarto, A.; Therigan, A.; Syakir, N.; Hidayat, S.; Risdiana, R. Blocking Temperature of Magnetite Nanoparticles Fe3O4 Encapsulated Silicon Dioxide SiO2. Key Eng. Mater. 2020, 855, 172–176. [Google Scholar] [CrossRef]
- Majetich, S.A. Magnetic Nanoparticles. In Handbook of Magnetism and Magnetic Materials; Coey, M., Parkin, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–36. [Google Scholar]
- Mohapatra, J.; Mitra, A.; Bahadur, D.; Aslam, M. Surface controlled fabrication of MFe2O4 (M = Mn, Fe, Co, Ni and Zn) nanoparticles and their magnetic properties. Cryst. Eng. Comm. 2012, 15, 524–532. [Google Scholar] [CrossRef]
- Nedelkoski, Z.; Kepaptsoglou, D.; Lari, L.; Wen, T.; Booth, R.A.; Oberdick, S.D.; Galindo, P.L.; Ramasse, Q.M.; Evans, R.F.L.; Majetich, S.; et al. Origin of reduced magnetization and domain formation in small magnetite nanoparticles. Sci. Rep. 2017, 7, 45997. [Google Scholar] [CrossRef] [Green Version]
- Guardia, P.; Labarta, A.; Batlle, X. Tuning the Size, the Shape, and the Magnetic Properties of Iron Oxide Nanoparticles. J. Phys. Chem. C 2011, 115, 390–396. [Google Scholar] [CrossRef]
- Mornet, S.; Vasseur, S.; Grasset, F.; Duguet, E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 2004, 14, 2161–2175. [Google Scholar] [CrossRef]
- Gazeau, F.; Levy, M.; Wilhelm, C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine 2008, 3, 831–844. [Google Scholar] [CrossRef]
- Natividad, E.; Castro, M.; Mediano, A. Adiabatic vs. non-adiabatic determination of specific absorption rate of ferrofluids. J. Magn. Magn. Mater. 2009, 321, 1497–1500. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Jeun, M.; Lee, S.; Kyeong Kang, J.; Tomitaka, A.; Wook Kang, K.; Il Kim, Y.; Takemura, Y.; Chung, K.-W.; Kwak, J.; Bae, S. Physical limits of pure superparamagnetic Fe3O4 nanoparticles for a local hyperthermia agent in nanomedicine. Appl. Phys. Lett. 2012, 100, 092406. [Google Scholar] [CrossRef] [Green Version]
- Filippousi, M.; Angelakeris, M.; Katsikini, M.; Paloura, E.; Efthimiopoulos, I.; Wang, Y.; Zamboulis, D.; Van Tendeloo, G. Surfactant Effects on the Structural and Magnetic Properties of Iron Oxide Nanoparticles. J. Phys. Chem. C 2014, 118, 16209–16217. [Google Scholar] [CrossRef]
- Wells, J.; Ortega, D.; Steinhoff, U.; Dutz, S.; Garaio, E.; Sandre, O.; Natividad, E.; Cruz, M.M.; Brero, F.; Southern, P.; et al. Challenges and recommendations for magnetic hyperthermia characterization measurements. Int. J. Hyperth. 2021, 38, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.A.-O.; Nalench, Y.A.-O.; Myrovali, E.; Garanina, A.S.; Grebennikov, I.S.; Gifer, P.K.; Abakumov, M.A.; Spasova, M.; Angelakeris, M.; Savchenko, A.G.; et al. Size-selected Fe(3)O(4)-Au hybrid nanoparticles for improved magnetism-based theranostics. Beilstein J. Nanotechnol. 2018, 9, 2684–2699. [Google Scholar] [CrossRef] [Green Version]
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, 1906539. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]






| Sample | Pd-Seeds (mg) | Heating Rate (°C/min) | [surf]/[Fe] (mol/mol) | Solvent | Average Diameter (nm ± SD) |
|---|---|---|---|---|---|
| Exp_standard | 20 | 5 | 5.5 | ODE | 19.5 ± 1.5 |
| Exp_10mg | 10 | 5 | 5.5 | ODE | 21.0 ± 2.0 |
| Exp_nDS | 20 | 5 | 5.5 | ODE | 14.7 ± 1.8 |
| Exp_3 °C | 20 | 3 | 5.5 | ODE | 13.3 ± 2.2 |
| Exp_7 °C | 20 | 7 | 5.5 | ODE | 18.9 ± 2.1 |
| Exp_[surf]/[Fe] = 2 | 20 | 5 | 2 | ODE | 9.8 ± 1.1 |
| Exp_[surf]/[Fe] = 3.25 | 20 | 5 | 3.25 | ODE | 16.1 ± 4.3 |
| Exp_[surf]/[Fe] = 11 | 20 | 5 | 11 | ODE | 16.3 ± 1.7 |
| Exp_OAm | 20 | 5 | 5.5 a | ODE | – c |
| Exp_OA | 20 | 5 | 5.5 b | ODE | – c |
| Exp_DPE | 20 | 5 | 5.5 | DPE | 12.1 ± 2.1 |
| Exp_ODE:DPE(1:1) | 20 | 5 | 5.5 | ODE:DPE | 15.5 ± 1.8 |
| Exp_DBE | 20 | 5 | 5.5 | DBE | 17.9 + 2.5 |
| Solvent | ODE | DBE | ODE:DPE (1:1) | DPE |
|---|---|---|---|---|
| Boiling point (°C) | 315 °C | 298 °C | ±270 °C | 258 °C |
| Average diameter (nm) | 19.5 ± 1.5 | 17.9 ± 2.5 | 15.5 + 1.8 | 12.1 ± 2.1 |
| Sample | Saturation Magnetization (Ms) (emu/g) | Coercivity (Hc) (Oe) | TB (K) | SLP a (W/gFe) | ILP (nHm2/kg) | r2 b (mM−1 s−1) | ||
|---|---|---|---|---|---|---|---|---|
| 5 K | 300 K | 5 K | 300 K | |||||
| Exp_standard | 67.9 | 58.7 | 20 | 8 | 180 | 113 | 0.975 | 220 |
| Exp_flower-like | 53.1 | 45.4 | 270 | 31 | 120 | -c | -c | -c |
| Exp_popcorn-like | 58.5 | 49.0 | 427 | 6 | >370 | -c | -c | -c |
| Exp_10 mg | 69.2 | 61.4 | 67 | 12 | 200 | 233 | 2.011 | 440 |
| Exp_nDS | 62.7 | 56.3 | 26 | 8 | 100 | -c | -c | -c |
| Exp_3 °C | 52.1 | 45.1 | 287 | 8 | 40 | -c | -c | -c |
| Exp_7 °C | 63.7 | 57.5 | 213 | 6 | 130 | 102 | 0.880 | 90 |
| Exp_[surf]/[Fe] = 2 | 26.4 | 20.5 | 466 | 10 | 30 | 2 | 0.017 | -c |
| Exp_[surf]/[Fe] = 11 | 62.4 | 55.8 | 81 | 4 | 120 | -c | -c | -c |
| Exp_DPE | 36.9 | 28.1 | 236 | 19 | 23 | -c | -c | -c |
| Exp_ODE:DPE (1:1) | 46.5 | 39.9 | 56 | 9 | 120 | -c | -c | -c |
| Exp_DBE | 63.6 | 55.4 | 13 | 4 | 160 | -c | -c | -c |
| Sample | Phase | IS (mm s−1) | QS (mm s−1) | Hyperfine Field (T) | Γ (mm s−1) | Spectral Contribution (%) |
|---|---|---|---|---|---|---|
| Exp_standard | Fe3+ (Fe3O4, A) | 0.32 | −0.01 | 47.2 | 0.46 | 24 |
| Fe2.5+δ (Fe3O4, B) | 0.54 | −0.01 | 43.3 | 1.03 | 76 | |
| Exp_flower-like | Fe3+ (Fe3O4, A) | 0.31 | −0.02 | 48.2 | 0.50 | 22 |
| Fe2.5+δ (Fe3O4, B) | 0.60 | 0.02 | 44.6 | 0.93 | 70 | |
| Fe3+ (SPM) | 0.19 | 0.98 | - | 0.95 | 8 | |
| Exp_10mg | Fe3+ (Fe3O4, A) | 0.34 | −0.02 | 47.5 | 0.57 | 38 |
| Fe2.5+δ (Fe3O4, B) | 0.50 | −0.01 | 43.2 | 1.12 | 62 | |
| Exp_nDS | Fe3+ (Fe3O4, A) | 0.33 | −0.01 | 47.8 | 0.53 | 41 |
| Fe2.5+δ (Fe3O4, B) | 0.49 | 0.02 | 43.7 | 0.99 | 59 | |
| Exp_7 °C | Fe3+ (Fe3O4, A) | 0.34 | −0.02 | 47.7 | 0.56 | 42 |
| Fe2.5+δ (Fe3O4, B) | 0.46 | −0.03 | 43.6 | 1.07 | 58 | |
| Exp_[surf]/[Fe] = 2 | Fe3+ (SPM) | 0.33 | 0.79 | - | 0.78 | 100 |
| Exp_ODE:DPE(1:1) | Fe3+ (SPM) | 0.36 | 0.80 | - | 0.74 | 41 |
| Fe3+ (γ-Fe2O3) | 0.34 | −0.02 | 45.6 | 0.68 | 59 | |
| Exp_DBE | Fe3+ (γ-Fe2O3) | 0.33 | −0.01 | 48.1 | 0.55 | 53 |
| Fe3+ (γ-Fe2O3) | 0.36 | −0.01 | 44.0 | 1.04 | 47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, A.; van Oossanen, R.; van Rhoon, G.C.; Pignol, J.-P.; Dugulan, I.; Denkova, A.G.; Djanashvili, K. From Structure to Function: Understanding Synthetic Conditions in Relation to Magnetic Properties of Hybrid Pd/Fe-Oxide Nanoparticles. Nanomaterials 2022, 12, 3649. https://doi.org/10.3390/nano12203649
Maier A, van Oossanen R, van Rhoon GC, Pignol J-P, Dugulan I, Denkova AG, Djanashvili K. From Structure to Function: Understanding Synthetic Conditions in Relation to Magnetic Properties of Hybrid Pd/Fe-Oxide Nanoparticles. Nanomaterials. 2022; 12(20):3649. https://doi.org/10.3390/nano12203649
Chicago/Turabian StyleMaier, Alexandra, Rogier van Oossanen, Gerard C. van Rhoon, Jean-Philippe Pignol, Iulian Dugulan, Antonia G. Denkova, and Kristina Djanashvili. 2022. "From Structure to Function: Understanding Synthetic Conditions in Relation to Magnetic Properties of Hybrid Pd/Fe-Oxide Nanoparticles" Nanomaterials 12, no. 20: 3649. https://doi.org/10.3390/nano12203649