Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations
Abstract
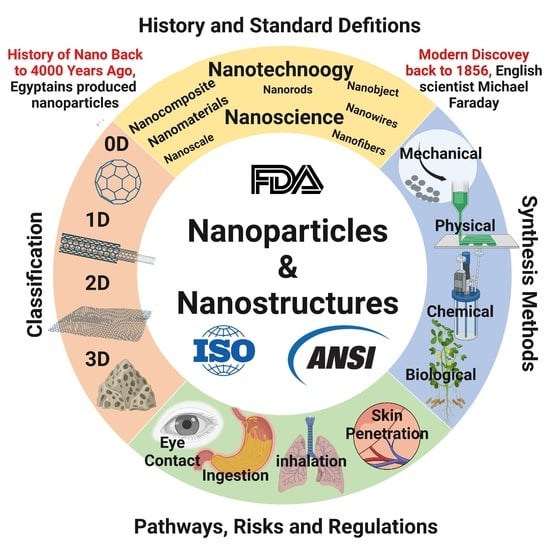
:1. Introduction
2. History and Development of Nanomaterial Research
2.1. Prehistoric Nanomaterials
2.2. Nanomaterials and Ancient Civilizations
2.3. Nanomaterials in Medieval Times
2.4. Nanomaterials in the Modern Age
2.5. Contemporary Nanomaterials in Material Engineering Applications
3. Nanomaterial Market Size
4. Nano Terminologies and Standard Definitions
5. Sources and Classifications of Nanomaterials
5.1. Nanomaterial Classification Based on Their Dimensionality
5.2. Nanomaterial Classification in the Function of Their Origin
5.3. Nanomaterial Classification as a Function of Their Chemical or Elemental Composition
5.4. Nanomaterial Classification as a Function of Their Porosity
5.5. Nanomaterial Classification as a Function of Their Crystallinity
5.6. Nanomaterial Classification as a Function of Their Dispersion
6. Nanofabrication and Engineering of Nanomaterials
6.1. Classification of Synthesis Methods Based on the Raw Materials
6.2. Classification Based on the Nature of the Deriving Forces
6.3. Classification Based on the Reaction Phase
7. Nanomaterial Features Are Influenced by Their Size and Shape
8. Naturally Occurring Nanomaterials
9. Life Cycle of the Nanomaterials and Environmental Risks
10. Toxicity of Nanomaterials
11. Nanomaterial Regulation and Challenges
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Kumar, V.; Kirubanandam, S.; Barhoum, A. Engineered nanomaterials: Nanofabrication and surface functionalization. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 305–340. ISBN 9780128135167. [Google Scholar]
- Hammani, S.; Barhoum, A.; Nagarajan, S.; Bechelany, M. Toner waste powder (twp) as a filler for polymer blends (LDPE/HIPS) for enhanced electrical conductivity. Materials 2019, 12, 3062. [Google Scholar] [CrossRef] [Green Version]
- Sudha, P.N.; Sangeetha, K.; Vijayalakshmi, K.; Barhoum, A. Nanomaterials history, classification, unique properties, production and market. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 341–384. ISBN 9780128135167. [Google Scholar]
- Liu, J.L.; Bashir, S. Advanced Nanomaterials and Their Applications in Renewable Energy; Elsevier Science: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Akpan, E.I.; Shen, X.; Wetzel, B.; Friedrich, K. Design and Synthesis of Polymer Nanocomposites. In Polymer Composites with Functionalized Nanoparticles: Synthesis, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 47–83. [Google Scholar]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Dai, Z.R.; Bradley, J.P.; Joswiak, D.J.; Brownlee, D.E.; Hill, H.G.M.; Genge, M.J. Possible in situ formation of meteoritic nanodiamonds in the early Solar System. Nature 2002, 418, 157–159. [Google Scholar] [CrossRef]
- Barhoum, A.; Luisa García-Betancourt, M. Physicochemical characterization of nanomaterials: Size, morphology, optical, magnetic, and electrical properties. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 279–304. ISBN 9780128135167. [Google Scholar]
- Barhoum, A.; García-Betancourt, M.L.; Rahier, H.; Van Assche, G. Physicochemical characterization of nanomaterials: Polymorph, composition, wettability, and thermal stability. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 255–278. ISBN 9780128135167. [Google Scholar]
- Aubert, M.; Brumm, A.; Ramli, M.; Sutikna, T.; Saptomo, E.W.; Hakim, B.; Morwood, M.J.; Van Den Bergh, G.D.; Kinsley, L.; Dosseto, A. Pleistocene cave art from Sulawesi, Indonesia. Nature 2014, 514, 223–227. [Google Scholar] [CrossRef]
- Youssef, A.M.; Moustafa, H.A.; Barhoum, A.; Hakim, A.E.-F.A.A.; Dufresne, A. Evaluation of the Morphological, Electrical and Antibacterial Properties of Polyaniline Nanocomposite Based on Zn/Al-Layered Double Hydroxides. ChemistrySelect 2017, 2. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Coskun, A.F.; England, G.; Cho, S.; Butt, H.; Hurwitz, J.; Kolle, M.; Khademhosseini, A.; Hart, A.J.; Folch, A.; et al. Art on the Nanoscale and beyond. Adv. Mater. 2016, 28, 1724–1742. [Google Scholar] [CrossRef] [Green Version]
- Heiligtag, F.J.; Niederberger, M. The fascinating world of nanoparticle research. Mater. Today 2013, 16, 262–271. [Google Scholar] [CrossRef]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Welcomme, E.; Hallégot, P.; Zaluzec, N.J.; Deeb, C.; Castaing, J.; Veyssière, P.; Bréniaux, R.; Lévêque, J.L.; Tsoucaris, G. Early Use of PbS Nanotechnology for an Ancient Hair Dyeing Formula. Nano Lett. 2006, 6, 2215–2219. [Google Scholar] [CrossRef]
- Johnson-Mcdaniel, D.; Barrett, C.A.; Sharafi, A.; Salguero, T.T. Nanoscience of an ancient pigment. J. Am. Chem. Soc. 2013, 135, 1677–1679. [Google Scholar] [CrossRef]
- Del Río, M.S.; Doménech, A.; Doménech-Carbó, M.T.; De Agredos Pascual, M.L.V.; Suárez, M.; García-Romero, E. The Maya Blue Pigment. Dev. Clay Sci. 2011, 3, 453–481. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Rendón, L.; Arenas, J.; Serra Puche, M.C. Maya Blue Paint: An Ancient Nanostructured Material. Science 1996, 273, 223–225. [Google Scholar] [CrossRef]
- Chiari, G.; Giustetto, R.; Ricchiardi, G. Crystal structure refinements of palygorskite and Maya Blue from molecular modelling and powder synchrotron diffraction. Eur. J. Mineral. 2003, 15, 21–33. [Google Scholar] [CrossRef]
- Schaming, D.; Remita, H. Nanotechnology: From the ancient time to nowadays. Found. Chem. 2015, 17, 187–205. [Google Scholar] [CrossRef]
- Artioli, G.; Angelini, I.; Polla, A. Crystals and phase transitions in protohistoric glass materials. Phase Transit. 2008, 81, 233–252. [Google Scholar] [CrossRef]
- Brun, N.; Mazerolles, L.; Pernot, M. Microstructure of opaque red glass containing copper. J. Mater. Sci. Lett. 1991, 10, 1418–1420. [Google Scholar] [CrossRef]
- Gettens, R.J. Maya Blue: An Unsolved Problem in Ancient Pigments. Am. Antiq. 1962, 27, 557–564. [Google Scholar] [CrossRef]
- Sanderson, K. Sharpest cut from nanotube sword. Nature 2006, 444, 286. [Google Scholar] [CrossRef]
- Reeve, J. The british museum. Cult. Educ. State 2019, 65–94. [Google Scholar] [CrossRef]
- Barber, D.J.; Freestone, I.C. An investigation of the origin of the colour of the lycurgus cup by analytical transmission electron microscopy. Archaeometry 1990, 32, 33–45. [Google Scholar] [CrossRef]
- Wagner, F.; Haslbeck, S.; Stievano, L.; Calogero, S.; Pankhurst, Q.; Martinek, K. Before striking gold in gold-ruby glass. Nature 2000, 407, 691–692. [Google Scholar] [CrossRef]
- Freestone, I.; Meeks, N.; Sax, M.; Higgitt, C. The Lycurgus Cup—A Roman nanotechnology. Gold Bull. 2007, 40, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Pradell, T.; Climent-Font, A.; Molera, J.; Zucchiatti, A.; Ynsa, M.D.; Roura, P.; Crespo, D. Metallic and nonmetallic shine in luster: An elastic ion backscattering study. J. Appl. Phys. 2007, 101, 103518. [Google Scholar] [CrossRef] [Green Version]
- Nakai, I.; Numako, C.; Hosono, H.; Yamasaki, K. Origin of the red color of satsuma copper-ruby glass as determined by EXAFS and optical absorption spectroscopy. J. Am. Ceram. Soc. 1999, 82, 689–695. [Google Scholar] [CrossRef]
- Bradbury, J. Nature’s Nanotechnologists: Unveiling the Secrets of Diatoms. PLoS Biol. 2004, 2, e306. [Google Scholar] [CrossRef] [Green Version]
- Barhoum, A.; Rahier, H.; Abou-Zaied, R.E.; Rehan, M.; Dufour, T.; Hill, G.; Dufresne, A. Effect of cationic and anionic surfactants on the application of calcium carbonate nanoparticles in paper coating. ACS Appl. Mater. Interfaces 2014, 6, 2734–2744. [Google Scholar] [CrossRef] [Green Version]
- Reibold, M.; Paufler, P.; Levin, A.A.; Kochmann, W.; Pätzke, N.; Meyer, D.C. Carbon nanotubes in an ancient Damascus sabre. Nature 2006, 444, 286. [Google Scholar] [CrossRef]
- Pérez-Arantegui, J.; Molera, J.; Larrea, A.; Pradell, T.; Vendrell-Saz, M.; Borgia, I.; Brunetti, B.G.; Cariati, F.; Fermo, P.; Mellini, M.; et al. Luster Pottery from the Thirteenth Century to the Sixteenth Century: A Nanostructured Thin Metallic Film. J. Am. Ceram. Soc. 2004, 84, 442–446. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [Green Version]
- Kheirandish, A.; Sepehri Javan, N.; Mohammadzadeh, H. Modified Drude model for small gold nanoparticles surface plasmon resonance based on the role of classical confinement. Sci. Rep. 2020, 10, 6517. [Google Scholar] [CrossRef] [Green Version]
- Rittner, M.N.; Abraham, T. Nanostructured materials: An overview and commercial analysis. Econ. Emerg. Technol. JOM 1998, 50, 37–38. [Google Scholar] [CrossRef]
- Niska, K.; Zielinska, E.; Radomski, M.W.; Inkielewicz-Stepniak, I. Metal nanoparticles in dermatology and cosmetology: Interactions with human skin cells. Chem. Biol. Interact. 2018, 295, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Brodusch, N.; Demers, H.; Gauvin, R. Field Emission Scanning Electron Microscopy; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Palmberg, Christopher & Nikulainen, Tuomo, 2006. “Industrial Renewal and Growth through Nanotechnology?—An Overview with Focus on Finland,” Discussion Papers 1020, The Research Institute of the Finnish Economy.
- Stephan, P.; Black, G.C.; Chang, T. The small size of the small scale market: The early-stage labor market for highly skilled nanotechnology workers. Res. Policy 2007, 36, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Hamid, H.A.; Jenidi, Y.; Thielemans, W.; Somerfield, C.; Gomes, R.L. Predicting the capability of carboxylated cellulose nanowhiskers for the remediation of copper from water using response surface methodology (RSM) and artificial neural network (ANN) models. Ind. Crops Prod. 2016, 93, 108–120. [Google Scholar] [CrossRef]
- Hulla, J.E.; Sahu, S.C.; Hayes, A.W. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef]
- Kaplan, S.; Radin, J. Bounding an emerging technology: Para-scientific media and the Drexler-Smalley debate about nanotechnology. Soc. Stud. Sci. 2011, 41, 457–485. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nanosci. Technol. 2009, 11–19. [Google Scholar] [CrossRef]
- Ruess, G.; Vogt, F. Höchstlamellarer Kohlenstoff aus Graphitoxyhydroxyd. Mon. Chem. Verwandte Teile And. Wiss. 1948, 78, 222–242. [Google Scholar] [CrossRef]
- Barhoum, A.; Rasouli, R.; Yousefzadeh, M.; Rahier, H.; Bechelany, M. Nanofiber Technologies: History and Development. In Handbook of Nanofibers; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 3–43. [Google Scholar]
- Abdel-Haleem, F.M.; Saad, M.; Barhoum, A.; Bechelany, M.; Rizk, M.S. PVC membrane, coated-wire, and carbon-paste ion-selective electrodes for potentiometric determination of galantamine hydrobromide in physiological fluids. Mater. Sci. Eng. C 2018, 89, 140–148. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A. Advances in Nanofibers for Antimicrobial Drug Delivery. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 733–774. [Google Scholar]
- Branstetter, L. Intellectual Property Rights, Innovation and Development: Is Asia Different? Millenn. Asia 2017, 8, 5–25. [Google Scholar] [CrossRef]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Gugulothu, D.; Barhoum, A.; Nerella, R.; Ajmer, R.; Bechlany, M. Fabrication of Nanofibers: Electrospinning and Non-Electrospinning Techniques. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–34. [Google Scholar]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; Madbouly, A.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. Molecularly Imprinted Electrochemical Sensor-Based Fe2O3@MWCNTs for Ivabradine Drug Determination in Pharmaceutical Formulation, Serum, and Urine Samples. Front. Bioeng. Biotechnol. 2021, 9, 213. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Mahmoud, S.; Abdel-Ghani, N.E.T.; El Nashar, R.M.; Bechelany, M.; Barhoum, A. Polyvinyl chloride modified carbon paste electrodes for sensitive determination of levofloxacin drug in serum, urine, and pharmaceutical formulations. Sensors 2021, 21, 3150. [Google Scholar] [CrossRef]
- El-Beshlawy, M.; Abdel-Haleem, F.; Barhoum, A. Molecularly Imprinted Polymer-Based Potentiometric Biosensor for Nanomolar Determination of Pioglitazone Hydrochloride in Pharmaceutical Formulations. Electroanalysis 2021, 33, 1244–1254. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. t-Butyl calixarene/Fe2O3@MWCNTs composite-based potentiometric sensor for determination of ivabradine hydrochloride in pharmaceutical formulations. Mater. Sci. Eng. C 2020, 116, 111110. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, F.M.; Salah, A.; Rizk, M.S.; Moustafa, H.; Bechelany, M.; Barhoum, A. Carbon-based Nanosensors for Salicylate Determination in Pharmaceutical Preparations. Electroanalysis 2019, 31, 778–789. [Google Scholar] [CrossRef]
- Hammani, S.; Barhoum, A.; Bechelany, M. Fabrication of PMMA/ZnO nanocomposite: Effect of high nanoparticles loading on the optical and thermal properties. J. Mater. Sci. 2018, 53, 1911–1921. [Google Scholar] [CrossRef]
- Omran, B.A. Fundamentals of Nanotechnology and Nanobiotechnology. In Nanobiotechnology: A Multidisciplinary Field of Science. Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Obeid, M.A.; Rezigue, M.M.; Alqudah, A.; Charbe, N.B.; Chellappan, D.K.; Mishra, V.; Pardhi, D.M.; Dureja, H.; Gupta, G.; et al. Nanocelluloses as a Novel Vehicle for Controlled Drug Delivery. In Handbook of Nanocelluloses; Barhoum, A., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Odenbach, S. Ferrofluids—Magnetically controlled suspensions. Colloids Surf. Physicochem. Eng. Asp. 2003, 217, 171–178. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticles—A historical perspective. Int. J. Pharm. 2007, 331, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, 1800256. [Google Scholar] [CrossRef] [PubMed]
- Karatutlu, A.; Barhoum, A.; Sapelkin, A. Liquid-phase synthesis of nanoparticles and nanostructured materials. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–28. ISBN 9780128135167. [Google Scholar]
- Berges, M.; Lum, M.R. A Global perspective on safe nanotechnology. In Proceedings of the XVIII World Congress on Safety and Health at Work, 30 June 2008; COEX Convention and Exhibition Center: Seoul, Korea, 2008. [Google Scholar]
- Sargent, J.J.F. The National Nanotechnology Initiative: Overview, Reauthorization, and Appropriations Issues; Congressional Research Service: Boca Raton, FL, USA, 2013. [Google Scholar]
- Fogelberg, H. Historical Context of the US National Nanotechnology Initiative. In Nano Meets Macro; Jenny Stanford Publishing: Singapore, 2019; pp. 29–53. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, S.; Chen, H.; Roco, M.C. International perspective on nanotechnology papers, patents, and NSF awards (2000–2016). J. Nanoparticle Res. 2017, 19, 1–11. [Google Scholar] [CrossRef]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Riego Sintes, J.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Shimada, Y.-A.; Tsukada, N.; Suzuki, J. Promoting diversity in science in Japan through mission-oriented research grants. Scientometrics 2017, 110, 1415–1435. [Google Scholar] [CrossRef]
- Khan, F.A. Nano-biotechnology. Biotechnol. Fundam. 2018, 421–444. [Google Scholar] [CrossRef]
- DeFrancesco, L. Little science, big bucks. Nat. Biotechnol. 2003, 21, 1127–1129. [Google Scholar] [CrossRef]
- Janković, N.Z.; Plata, D.L. Engineered nanomaterials in the context of global element cycles. Environ. Sci. Nano 2019, 6, 2697–2711. [Google Scholar] [CrossRef] [Green Version]
- Bostrom, A.; Löfstedt, R. Nanotechnology Risk Communication. Nanotechnology 2017, 215–230. [Google Scholar] [CrossRef]
- Bubakir, M.M.; Li, H.; Barhoum, A.; Yang, W. Advances in Melt Electrospinning Technique. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Babick, F.; Mielke, J.; Wohlleben, W.; Weigel, S.; Hodoroaba, V.D. How reliably can a material be classified as a nanomaterial? Available particle-sizing techniques at work. J. Nanoparticle Res. 2016, 18, 1–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, C.A.; Stinz, M.; Hodoroaba, V.D.; Unger, W.E.S.; Fujimoto, T. International standards in nanotechnologies. Charact. Nanoparticles Meas. Process. Nanoparticles 2020, 511–525. [Google Scholar] [CrossRef]
- Klaessig, F.; Marrapese, M.; Abe, S. Current Perspectives in Nanotechnology Terminology and Nomenclature. In Nanotechnology standards; Springer: New York, NY, USA, 2011; pp. 21–52. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Barhoum, A.; El-Sherbiny, S.; Morsy, F.; El-Midany, A.A.-H.; Rahier, H. Preparation of superhydrophobic nanocalcite crystals using Box–Behnken design. Arab. J. Chem. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Soltani, A.M.; Pouypouy, H. Standardization and Regulations of Nanotechnology and Recent Government Policies Across the World on Nanomaterials. In Advances in Phytonanotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 419–446. [Google Scholar] [CrossRef]
- Kallinger, C.; Veffkind, V.; Michalitsch, R.; Verbandt, Y. Patenting Nanotechnology: A European Patent Office Perspective. Nanotechnol. Law Bus. 2008, 5, 95. [Google Scholar]
- Hammani, S.; Moulai-Mostefa, N.; Samyn, P.; Bechelany, M.; Dufresne, A.; Barhoum, A. Morphology, Rheology and Crystallization in Relation to the Viscosity Ratio of Polystyrene/Polypropylene Polymer Blends. Materials 2020, 13, 926. [Google Scholar] [CrossRef] [Green Version]
- Bowman, D.M.; May, N.D.; Maynard, A.D. Nanomaterials in Cosmetics: Regulatory Aspects. In Analysis of Cosmetic Products, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 289–302. [Google Scholar] [CrossRef]
- Kazakov, S.; Ruiz-Alba, J.L. The impact of information and communication technology and internal market orientation blending on organisational performance in small and medium enterprises. Eur. J. Manag. Bus. Econ. 2020. [Google Scholar] [CrossRef]
- Atanassov, A.; Trifonova, S.; Saraivanova, J.; Pramatarov, A.; Atanassov, A.; Trifonova, S.; Saraiva-nova, J.; Pramatarov, A. Assessment of the administrative burdens for businesses in Bulgaria according to the national legislation related to the European Union internal market. Manag. J. Contemp. Manag. Issues 2017, 22, 21–49. [Google Scholar]
- Hischier, R.; Walser, T. Life cycle assessment of engineered nanomaterials: State of the art and strategies to overcome existing gaps. Sci. Total Environ. 2012, 425, 271–282. [Google Scholar] [CrossRef]
- Rauscher, H.; Rasmussen, K.; Sokull-Klüttgen, B. Regulatory Aspects of Nanomaterials in the EU. Chem. Ing. Tech. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, A.; Dhawan, A. Nanomaterials: Exposure, Effects and Toxicity Assessment. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2012, 82, 3–11. [Google Scholar] [CrossRef]
- Slezakova, K.; Morais, S.; Do, M.; Pereira, C. Atmospheric Nanoparticles and Their Impacts on Public Health. In Current Topics in Public Health; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Dahm, M.M.; Schubauer-Berigan, M.K.; Evans, D.E.; Birch, M.E.; Bertke, S.; Beard, J.D.; Erdely, A.; Fernback, J.E.; Mercer, R.R.; Grinshpun, S.A. Exposure assessments for a cross-sectional epidemiologic study of US carbon nanotube and nanofiber workers. Int. J. Hyg. Environ. Health 2018, 221, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Zidan, S.; Silikas, N.; Alhotan, A.; Haider, J.; Yates, J. Investigating the Mechanical Properties of ZrO2-Impregnated PMMA Nanocomposite for Denture-Based Applications. Materials 2019, 12, 1344. [Google Scholar] [CrossRef] [Green Version]
- Bathi, J.R.; Moazeni, F.; Upadhyayula, V.K.K.; Chowdhury, I.; Palchoudhury, S.; Potts, G.E.; Gadhamshetty, V. Behavior of engineered nanoparticles in aquatic environmental samples: Current status and challenges. Sci. Total Environ. 2021, 793, 148560. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ke, C. Structural and physical properties of boron nitride nanotubes and their applications in nanocomposites. In Boron Nitride Nanotubes in Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Salama, A.; Abouzeid, R.; Leong, W.S.; Jeevanandam, J.; Samyn, P.; Dufresne, A.; Bechelany, M.; Barhoum, A. Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration. Nanomaterials 2021, 11, 3008. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Favre, T.; Sayegh, S.; Tanos, F.; Coy, E.; Iatsunskyi, I.; Razzouk, A.; Cretin, M.; Bechelany, M. 3D Self-Supported Nitrogen-Doped Carbon Nanofiber Electrodes Incorporated Co/CoOx Nanoparticles: Application to Dyes Degradation by Electro-Fenton-Based Process. Nanomaterials 2021, 11, 2686. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Sundaramurthy, A.; Sharma, V.; Murugan, C.; Pal, K.; Kodous, M.H.A.; Danquah, M.K. Sustainability of One-Dimensional Nanostructures: Fabrication and Industrial Applications. In Sustainable Nanoscale Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 83–113. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Barhoum, A.; Nada, A.A.; Moustafa, Y.M.; Seliman, S.M.; Youssef, A.M.; Bechelany, M. Synthesis of mesoporous core-shell CdS@TiO2 (0D and 1D) photocatalysts for solar-driven hydrogen fuel production. J. Photochem. Photobiol. Chem. 2018, 351, 261–270. [Google Scholar] [CrossRef]
- Barhoum, A.; El-Maghrabi, H.H.; Nada, A.A.; Sayegh, S.; Roualdes, S.; Renard, A.; Iatsunskyi, I.; Coy, E.; Bechelany, M. Simultaneous hydrogen and oxygen evolution reactions using free-standing nitrogen-doped-carbon–Co/CoOx nanofiber electrodes decorated with palladium nanoparticles. J. Mater. Chem. 2021, 9, 17724–17739. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Li, Y.; Smekens, J.; Barhoum, A.; Van Assche, G.; Omar, N.; Van Mierlo, J. Electrochemical impedance spectroscopy characterization and parameterization of lithium nickel manganese cobalt oxide pouch cells: Dependency analysis of temperature and state of charge. Ionics 2019, 25, 111–123. [Google Scholar] [CrossRef]
- Zhao, H.; Lei, Y. 3D Nanostructures for the Next Generation of High-Performance Nanodevices for Electrochemical Energy Conversion and Storage. Adv. Energy Mater. 2020, 10, 2001460. [Google Scholar] [CrossRef]
- Nnaji, C.O.; Jeevanandam, J.; Chan, Y.S.; Danquah, M.K.; Pan, S.; Barhoum, A. Engineered nanomaterials for wastewater treatment: Current and future trends. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–168. [Google Scholar]
- Ho, D.T.; Ho, V.H.; Babar, V.; Kim, S.Y.; Schwingenschlögl, U. Complex three-dimensional graphene structures driven by surface functionalization. Nanoscale 2020, 12, 10172–10179. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.H.; Yang, H.Y.; Huang, H.Q.; Chen, G.X.; Zheng, N.F. Solvent effect on the synthesis of monodisperse amine-capped Au nanoparticles. Chin. Chem. Lett. 2013, 24, 457–462. [Google Scholar] [CrossRef]
- Rahmani, S.; Budimir, J.; Sejalon, M.; Daurat, M.; Aggad, D.; Vivès, E.; Raehm, L.; Garcia, M.; Lichon, L.; Gary-Bobo, M.; et al. Large Pore Mesoporous Silica and Organosilica Nanoparticles for Pepstatin a Delivery in Breast Cancer Cells. Molecules 2019, 24, 332. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Langille, M.R.; Mirkin, C.A. Synthesis of silver nanorods by low energy excitation of spherical plasmonic seeds. Nano Lett. 2011, 11, 2495–2498. [Google Scholar] [CrossRef]
- Guo, K.W.; Tam, H.-Y. TEM Morphology of Carbon Nanotubes (CNTs) and its Effect on the Life of Micropunch. Transm. Electron Microsc.—Theory Appl. 2015. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Q.; Wang, F.X.; Xiao, Y.; Peng, H.D.; Zhong, H.J.; Liu, Z.H.; Pan, G.B. Facile Microwave-Assisted Synthesis of Klockmannite CuSe Nanosheets and Their Exceptional Electrical Properties. Sci. Rep. 2014, 4, 5998. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Adamcik, J.; Mezzenga, R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nat. Nanotechnol. 2012, 7, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Obeid, M.A.; Zoubi, M.S.A.; Charbe, N.B.; Chellappan, D.K.; Mishra, V.; Dureja, H.; Gupta, G.; Prasher, P.; Dua, K.; et al. Nanocelluloses in Sensing Technology. In Handbook of Nanocelluloses; Barhoum, A., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Qian, G.; Peng, Q.; Zou, D.; Wang, S.; Yan, B. Hydrothermal Synthesis of Flake-Flower NiO and Its Gas Sensing Performance to CO. Front. Mater. 2020, 7, 216. [Google Scholar] [CrossRef]
- Ngô, C.; Van De Voorde, M. Nanotechnology in a Nutshell: From Simple to Complex Systems; Springer Nature: Cham, Switzerland, 2014; Volume 9789462390, ISBN 9789462390126. [Google Scholar]
- Shalan, A.E.; Barhoum, A.; Elseman, A.M.; Rashad, M.M.; Lira-Cantú, M. Nanofibers as Promising Materials for New Generations of Solar Cells. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Teyssier, J.; Saenko, S.V.; Van Der Marel, D.; Milinkovitch, M.C. Photonic crystals cause active colour change in chameleons. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.H.; Choi, T.M.; Kim, B.; Han, S.H.; Lee, J.M.; Kim, S.H. Chameleon-Inspired Mechanochromic Photonic Films Composed of Non-Close-Packed Colloidal Arrays. ACS Nano 2017, 11, 11350–11357. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef]
- Samyn, P.; Barhoum, A. Engineered nanomaterials for papermaking industry. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 245–277. [Google Scholar]
- Albalawi, F.; Hussein, M.Z.; Fakurazi, S.; Masarudin, M.J. Engineered nanomaterials: The challenges and opportunities for nanomedicines. Int. J. Nanomed. 2021, 16, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugulothu, D.; Barhoum, A.; Afzal, S.M.; Venkateshwarlu, B.; Uludag, H. Structural Multifunctional Nanofibers and their Emerging Applications. In Handbook of Nanofibers; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–41. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.E.; Sumant, A.V. The CVD of nanodiamond materials. Chem. Vap. Depos. 2008, 14, 145–160. [Google Scholar] [CrossRef]
- Barhoum, A.; El-Maghrabi, H.H.; Iatsunskyi, I.; Coy, E.; Renard, A.; Salameh, C.; Weber, M.; Sayegh, S.; Nada, A.A.; Roualdes, S.; et al. Atomic Layer Deposition of Pd Nanoparticles on Self-Supported Carbon-Ni/NiO-Pd Nanofiber Electrodes for Electrochemical Hydrogen and Oxygen Evolution Reactions. J. Colloid Interface Sci. 2020, 569, 286–297. [Google Scholar] [CrossRef]
- Oschatz, M.; Borchardt, L.; Hippauf, F.; Nickel, W.; Kaskel, S.; Brunner, E. Interactions between Electrolytes and Carbon-Based Materials—NMR Studies on Electrical Double-Layer Capacitors, Lithium-Ion Batteries, and Fuel Cells. In Annual Reports on NMR Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2016; Volume 87, pp. 237–318. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ramsden, J.; Ramsden, J.J. The impact of nanotechnology The impacts of nanotechnology. Nanotechnol. Percept. 2011, 7, 28–66. [Google Scholar] [CrossRef]
- Stevens, B.; Etherington, N. Quantum Dots Quantum Dots. Nanoparticles Biomed. Appl. 2019, 1072, 1–36. [Google Scholar]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef]
- Essawy, H.; El-Sabbagh, S.; Tawfik, M.; Bulletin, G.V.A.-P. Assessment of Provoked Compatibility of NBR/SBR Polymer Blend with Montmorillonite Amphiphiles from the Thermal Degradation Kinetics; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Rodríguez-Hernández, J. Nanostructured antimicrobial materials in the food industry. In Food Preservation; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Siddiqui, S.I.; Fatima, B.; Tara, N.; Rathi, G.; Chaudhry, S.A. Recent advances in remediation of synthetic dyes from wastewaters using sustainable and low-cost adsorbents. In The Impact and Prospects of Green Chemistry for Textile Technology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 471–507. ISBN 9780081024911. [Google Scholar]
- Neitzel, I.; Mochalin, V.; Gogotsi, Y. Advances in Surface Chemistry of Nanodiamond and Nanodiamond–Polymer Composites. Ultrananocrystalline Diam. Synth. Prop. Appl. 2012, 421–456. [Google Scholar] [CrossRef]
- Zhang, C. Understanding the wear and tribological properties of ceramic matrix composites. In Advances in Ceramic Matrix Composites; Elsevier: Amsterdam, The Netherlands, 2014; pp. 312–339. ISBN 9780857091208. [Google Scholar]
- Saboori, A.; Dadkhah, M.; Fino, P.; Pavese, M. An overview of metal matrix nanocomposites reinforced with graphene nanoplatelets; mechanical, electrical and thermophysical properties. Metals 2018, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Rui, L. Reis Encyclopedia of Tissue Engineering and Regenerative Medicine—Google Libros; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Barhoum, A.; Van Assche, G.; Rahier, H.; Fleisch, M.; Bals, S.; Delplancked, M.-P.; Leroux, F.; Bahnemann, D. Sol-gel hot injection synthesis of ZnO nanoparticles into a porous silica matrix and reaction mechanism. Mater. Des. 2017, 119, 270–276. [Google Scholar] [CrossRef]
- Barhoum, A.; Melcher, J.; Van Assche, G.; Rahier, H.; Bechelany, M.; Fleisch, M.; Bahnemann, D. Synthesis, growth mechanism, and photocatalytic activity of Zinc oxide nanostructures: Porous microparticles versus nonporous nanoparticles. J. Mater. Sci. 2017, 52, 2746–2762. [Google Scholar] [CrossRef]
- Karatutlu, A.; Barhoum, A.; Sapelkin, A. Theories of nanoparticle and nanostructure formation in liquid phase. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 597–619. ISBN 9780128135167. [Google Scholar]
- Wang, B.; Prinsen, P.; Wang, H.; Bai, Z.; Wang, H.; Luque, R.; Xuan, J. Macroporous materials: Microfluidic fabrication, functionalization and applications. Chem. Soc. Rev. 2017, 46, 855–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanuel, T.; Mishra, M. Comparative Study of Thermal and Hydraulic Performance of Three-Fluid Tubular Heat Exchanger with CuO–Water Nanofluid: Single-Phase and Multi-Phase Approaches. J. Therm. Sci. Eng. Appl. 2021, 13, 031012. [Google Scholar] [CrossRef]
- Illath, K.; Narasimahan, A.K.; Nagai, M.; Wankhar, S.; Santra, T.S. Microfluidics-Based Metallic Nanoparticle Synthesis and Applications. In Microfluidics and Bio-MEMS; Jenny Stanford Publishing: Singapore, 2020; pp. 429–501. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Strojan, K.; Leonardi, A.; Bregar, V.B.; Križaj, I.; Svete, J.; Pavlin, M. Dispersion of Nanoparticles in Different Media Importantly Determines the Composition of Their Protein Corona. PLoS ONE 2017, 12, e0169552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Woodhead Publishing: Cambridge, UK, 2017; pp. 43–58. [Google Scholar] [CrossRef]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res. 2013, 15, 1–17. [Google Scholar] [CrossRef]
- Bai, W.; Ross, C.A. Functional nanostructured materials based on self-assembly of block copolymers. MRS Bull. 2016, 41, 100–107. [Google Scholar] [CrossRef]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. Supramol. Chem. 2012. [Google Scholar] [CrossRef]
- Patel, J.K.; Patel, A.; Bhatia, D. Introduction to Nanomaterials and Nanotechnology. In Emerging Technologies for Nanoparticle Manufacturing 2021; Springer: Cham, Switzerland, 2021; pp. 3–23. [Google Scholar] [CrossRef]
- Schmidt, O.G.; Deneke, C.; Nakamura, Y.; Zapf-Gottwick, R.; Müller, C.; Jin-Phillipp, N.Y. Nanotechnology—Bottom-up Meets Top-down. In Advances in Solid State Physics; Springer: Berlin/Heidelberg, Germnay, 2002; pp. 231–240. [Google Scholar] [CrossRef]
- Ranjan, S.; Dasgupta, N.; Rajendran, B.; Avadhani, G.S.; Ramalingam, C.; Kumar, A. Microwave-irradiation-assisted hybrid chemical approach for titanium dioxide nanoparticle synthesis: Microbial and cytotoxicological evaluation. Environ. Sci. Pollut. Res. 2016, 23, 12287–12302. [Google Scholar] [CrossRef]
- Marcelo, G.A.; Lodeiro, C.; Capelo, J.L.; Lorenzo, J.; Oliveira, E. Magnetic, fluorescent and hybrid nanoparticles: From synthesis to application in biosystems. Mater. Sci. Eng. C 2020, 106, 110104. [Google Scholar] [CrossRef]
- Hoang, V.V. Atomic mechanism of vitrification process in simple monatomic nanoparticles. Eur. Phys. J. D 2011, 61, 627–635. [Google Scholar] [CrossRef]
- Kadhum Alghanimi, S.M.; Hadi, S.S. The environmental effects of nano powder against some microbes that isolated from oral cavity. IOP Conf. Ser. Earth Environ. Sci. 2021, 790, 012067. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Sharma, V.; Kala, P. Sustainable techniques in grinding: State of the art review. J. Clean. Prod. 2020, 269, 121876. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Cui, L.; Yan, L.; Zhao, C.; Dong, Y. Cold condensing scrubbing method for fine particle reduction from saturated flue gas. Energy 2019, 171, 1193–1205. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Barhoum, A.; Samyn, P.; Öhlund, T.; Dufresne, A. Review of recent research on flexible multifunctional nanopapers. Nanoscale 2017, 9, 15181–15205. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ahijón, E.; Marín-Gamero, R.; Molero-Sánchez, B.; Ávila-Brande, D.; Manjón-Sanz, A.; Fernández-Díaz, M.T.; Morán, E.; Schmidt, R.; Prado-Gonjal, J. From theory to experiment: BaFe 0.125 Co 0.125 Zr 0.75 O 3−δ, a highly promising cathode for intermediate temperature SOFCs. J. Mater. Chem. A 2020, 8, 3413–3420. [Google Scholar] [CrossRef]
- Belaiche, Y.; Khelef, A.; Laouini, S.E.; Bouafia, A.; Tedjani, M.L.; Barhoum, A. Green Synthesis and Characterization of Silver/Silver Oxide Nanoparticles Using Aqueous Leaves Extract of Artemisia Herba-Alba As Reducing And Capping Agents. Rev. Română Mater./Rom. J. Mater. 2021, 51, 342–352. [Google Scholar]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Janas, D.; Koziol, K.K. Carbon nanotube fibers and films: Synthesis, applications and perspectives of the direct-spinning method. Nanoscale 2016, 8, 19475–19490. [Google Scholar] [CrossRef] [PubMed]
- Pesheck, P.; Lorence, M. (Eds.) Development of Packaging and Products for Use in Microwave Ovens; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Panigrahi, S.; Kundu, S.; Ghosh, S.K.; Nath, S.; Pal, T. General method of synthesis for metal nanoparticles. J. Nanoparticle Res. 2004, 6, 411–414. [Google Scholar] [CrossRef]
- Nikam, A.V.; Prasad, B.L.V.; Kulkarni, A.A. Wet chemical synthesis of metal oxide nanoparticles: A review. CrystEngComm 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Handbook of Nanofibers, 1st ed.; Barhoum, A.; Bechelany, M.; Makhlouf, A.S.H. (Eds.) Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-319-53654-5. [Google Scholar]
- Tadic, M.; Trpkov, D.; Kopanja, L.; Vojnovic, S.; Panjan, M. Hydrothermal synthesis of hematite (α-Fe2O3) nanoparticle forms: Synthesis conditions, structure, particle shape analysis, cytotoxicity and magnetic properties. J. Alloy. Compd. 2019, 792, 599–609. [Google Scholar] [CrossRef]
- Che, G.; Lakshmi, B.B.; Martin, C.R.; Fisher, E.R.; Ruoff, R.S. Chemical Vapor Deposition Based Synthesis of Carbon Nanotubes and Nanofibers Using a Template Method. Chem. Mater. 1998, 10, 260–267. [Google Scholar] [CrossRef]
- Jhung, S.H.; Jin, T.; Hwang, Y.K.; Chang, J.S. Microwave Effect in the Fast Synthesis of Microporous Materials: Which Stage between Nucleation and Crystal Growth is Accelerated by Microwave Irradiation? Chem.—A Eur. J. 2007, 13, 4410–4417. [Google Scholar] [CrossRef]
- Campos, E.A.; Pinto, D.V.B.S.; de Oliveira, J.I.S.; Mattos, E.D.C.; Dutra, R.D.C.L. Synthesis, Characterization and Applications of Iron Oxide Nanoparticles—A Short Review. J. Aerosp. Technol. Manag. 2015, 7, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Gonzalo, J.; Perea, A.; Babonneau, D.; Afonso, C.N.; Beer, N.; Barnes, J.P.; Petford-Long, A.K.; Hole, D.E.; Townsend, P.D. Competing processes during the production of metal nanoparticles by pulsed laser deposition. Phys. Rev. B—Condens. Matter Mater. Phys. 2005, 71, 125420. [Google Scholar] [CrossRef] [Green Version]
- Wani, T.U.; Pandith, A.H.; Sheikh, F.A. Polyelectrolytic nature of chitosan: Influence on physicochemical properties and synthesis of nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 65, 102730. [Google Scholar] [CrossRef]
- Komal, S.; Kukreti, S.; Kaushik, M. Exploring the potential of environment friendly silver nanoparticles for DNA interaction: Physicochemical approach. J. Photochem. Photobiol. B Biol. 2019, 194, 158–165. [Google Scholar] [CrossRef]
- Barhoum, A.; Ibrahim, H.M.; Hassanein, T.F.; Hill, G.; Reniers, F.; Dufour, T.; Delplancke, M.P.; Van Assche, G.; Rahier, H. Preparation and characterization of ultra-hydrophobic calcium carbonate nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012037. [Google Scholar] [CrossRef] [Green Version]
- Parsons, J.G.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Chapter 21 Use of plants in biotechnology: Synthesis of metal nanoparticles by inactivated plant tissues, plant extracts, and living plants. Dev. Environ. Sci. 2007, 5, 463–485. [Google Scholar] [CrossRef]
- Willner, I.; Baron, R.; Willner, B. Growing metal nanoparticles by enzymes. Adv. Mater. 2006, 18, 1109–1120. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of Metal and Metal Oxide Nanoparticles. ChemBioEng Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- Gas-Phase Synthesis of Nanoparticles; John Wiley & Sons: Hoboken, NJ, USA, 2017.
- Barhoum, A.; Rahier, H.; Benelmekki, M.; Assche, G. Van Recent trends in nanostructured particles: Synthesis, functionalization, and applications. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 605–639. [Google Scholar]
- Katouah, H.; El-Metwaly, N.M. Plasma treatment toward electrically conductive and superhydrophobic cotton fibers by in situ preparation of polypyrrole and silver nanoparticles. React. Funct. Polym. 2021, 159, 104810. [Google Scholar] [CrossRef]
- Thimsen, E.; Kortshagen, U.R.; Aydil, E.S. Plasma synthesis of stoichiometric Cu2S nanocrystals stabilized by oleylamine. Chem. Commun. 2014, 50, 8346–8349. [Google Scholar] [CrossRef]
- Chang, I. Plasma synthesis of metal nanopowders. In Advances in Powder Metallurgy; Woodhead Publishing: Cambridge, UK, 2013; pp. 69–85. [Google Scholar] [CrossRef]
- Mantzaris, N.V. Liquid-phase synthesis of nanoparticles: Particle size distribution dynamics and control. Chem. Eng. Sci. 2005, 60, 4749–4770. [Google Scholar] [CrossRef]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443–455. [Google Scholar] [CrossRef]
- Nalluri, S.R.; Nagarjuna, R.; Patra, D.; Ganesan, R.; Balaji, G. Large Scale Solid-state Synthesis of Catalytically Active Fe3O4@M (M = Au, Ag and Au-Ag alloy) Core-shell Nanostructures. Sci. Rep. 2019, 9, 6603. [Google Scholar] [CrossRef]
- Ray, P.C. Size and shape dependent second order nonlinear optical properties of nanomaterials and their application in biological and chemical sensing. Chem. Rev. 2010, 110, 5332–5365. [Google Scholar] [CrossRef] [Green Version]
- Turky, A.O.; Barhoum, A.; MohamedRashad, M.; Bechlany, M. Enhanced the structure and optical properties for ZnO/PVP nanofibers fabricated via electrospinning technique. J. Mater. Sci. Mater. Electron. 2017, 28, 17526–17532. [Google Scholar] [CrossRef]
- Cremers, V.; Rampelberg, G.; Barhoum, A.; Walters, P.; Claes, N.; Oliveira, T.M.D.; Assche, G.V.; Bals, S.; Dendooven, J.; Detavernier, C. Oxidation barrier of Cu and Fe powder by Atomic Layer Deposition. Surf. Coat. Technol. 2018, 349, 1032–1041. [Google Scholar] [CrossRef]
- Shin, W.K.; Cho, J.; Kannan, A.G.; Lee, Y.S.; Kim, D.W. Cross-linked Composite Gel Polymer Electrolyte using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries. Sci. Rep. 2016, 6, 26332. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Van Assche, G.; Makhlouf, A.S.H.; Terryn, H.; Baert, K.; Delplancke, M.-P.; El-Sheikh, S.M.; Rahier, H. A Green, Simple Chemical Route for the Synthesis of Pure Nanocalcite Crystals. Cryst. Growth Des. 2015, 15, 573–580. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Yan, Y.; Huang, J.; Petukhov, A.V.; Kroon-Batenburg, L.M.J.; Drechsler, M.; Zhou, C.; Tu, M.; Granick, S.; Jiang, L. Giant capsids from lattice self-assembly of cyclodextrin complexes. Nat. Commun. 2017, 8, 15856. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, F.; Panahi, F.; Daneshgar, F.; Yousefi, R.; Shahsavani, M.B.; Khalafi-Nezhad, A. Synthesis of new α-aminophosphonate derivatives incorporating benzimidazole, theophylline and adenine nucleobases using l -cysteine functionalized magnetic nanoparticles (LCMNP) as magnetic reusable catalyst: Evaluation of their anticancer properties. RSC Adv. 2016, 6, 5915–5924. [Google Scholar] [CrossRef]
- Han, S.; Meng, Q.; Araby, S.; Liu, T.; Demiral, M. Mechanical and electrical properties of graphene and carbon nanotube reinforced epoxy adhesives: Experimental and numerical analysis. Compos. Part A Appl. Sci. Manuf. 2019, 120, 116–126. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, L.; Li, H.; Barhoum, A.; Zhang, Y.; He, X.; Yang, W.; Bubakir, M.M.; Chen, H. Magnetic Nanofibers: Unique Properties, Fabrication Techniques, and Emerging Applications. ChemistrySelect 2018, 3, 9127–9143. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor Nanocrystals: Structure, Properties, and Band Gap Engineering. Acc. Chem. Res. 2009, 43, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Rehan, M.; Barhoum, A.; Khattab, T.A.; Gätjen, L.; Wilken, R. Colored, photocatalytic, antimicrobial and UV-protected viscose fibers decorated with Ag/Ag2CO3 and Ag/Ag3PO4 nanoparticles. Cellulose 2019, 26, 5437–5453. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Sawy, A.M.; Barhoum, A.; Abdel Gaber, S.A.; El-Hallouty, S.M.; Shousha, W.G.; Maarouf, A.A.; Khalil, A.S.G. Insights of doxorubicin loaded graphene quantum dots: Synthesis, DFT drug interactions, and cytotoxicity. Mater. Sci. Eng. C 2021, 122, 111921. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles—Formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, K.X.; Barhoum, A.; Pan, S.; Danquah, M.K. Risks and toxicity of nanoparticles and nanostructured materials. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. ISBN 9780128135167. [Google Scholar]
- Olins, D.E.; Olins, A.L. Chromatin history: Our view from the bridge. Nat. Rev. Mol. Cell Biol. 2003, 4, 809–814. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.H.; Liu, M.; Nolting, B.; Go, J.G.; Gervay-Hague, J.; Liu, G.Y. A Nanoengineering Approach for Investigation and Regulation of Protein Immobilization. ACS Nano 2008, 2, 2374–2384. [Google Scholar] [CrossRef] [Green Version]
- Noor, Z. Nanohydroxyapatite application to osteoporosis management. J. Osteoporos. 2013, 2013, 679025. [Google Scholar] [CrossRef] [Green Version]
- Demirel, Y. Nonequilibrium thermodynamics modeling of coupled biochemical cycles in living cells. J. Nonnewton. Fluid Mech. 2010, 165, 953–972. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, Y.; Wang, Y.; Shu, Y.; Tan, H.; Zhao, Y. Bioinspired structural color particles with multi-layer graphene oxide encapsulated nanoparticle components. Bioact. Mater. 2020, 5, 917–923. [Google Scholar] [CrossRef]
- Yin, H.; Dong, B.; Liu, X.; Zhan, T.; Shi, L.; Zi, J.; Yablonovitch, E. Amorphous diamond-structured photonic crystal in the feather barbs of the scarlet macaw. Proc. Natl. Acad. Sci. USA 2012, 109, 10798–10801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, T. Biomimetics: Design by nature. Natl. Geogr. Mag. 2008, 213, 68–91. [Google Scholar]
- Xiao, M.; Shawkey, M.D.; Dhinojwala, A. Bioinspired Melanin-Based Optically Active Materials. Adv. Opt. Mater. 2020, 8, 2000932. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, C.; Li, J.; Wei, J.; Guo, J. A color-changing plasmonic actuator based on silver nanoparticle array/liquid crystalline elastomer nanocomposites. New J. Chem. 2016, 40, 7311–7319. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, C.; Li, J.; Liu, Y.; Zheng, Y.; Guo, Z.; Gao, C.; Li, J.; Liu, Y.; Zheng, Y. Antiicing Properties of Bioinspired Liquid-Infused Double-Layer Surface with Internal Wetting Transport Ability. Adv. Mater. Interfaces 2019, 6, 1900244. [Google Scholar] [CrossRef]
- Nakanishi, T.; Hiraoka, T.; Fujimoto, A.; Okino, T.; Sugimura, S.; Shimada, T.; Asakawa, K. Large Area fabrication of moth-eye antireflection structures using self-assembled nanoparticles in combination with nanoimprinting. Jpn. J. Appl. Phys. 2010, 49, 0750011–0750017. [Google Scholar] [CrossRef]
- Mu, Z.; Zhao, X.; Xie, Z.; Zhao, Y.; Zhong, Q.; Bo, L.; Gu, Z. In situ synthesis of gold nanoparticles (AuNPs) in butterfly wings for surface enhanced Raman spectroscopy (SERS). J. Mater. Chem. B 2013, 1, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Wilts, B.D.; Saranathan, V.; Wilts, B.D.; Saranathan, V. A Literal Elytral Rainbow: Tunable Structural Colors Using Single Diamond Biophotonic Crystals in Pachyrrhynchus congestus Weevils. Small 2018, 14, 1802328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vali, H.; Förster, O.; Amarantidis, G.; Petersen, N. Magnetotactic bacteria and their magnetofossils in sediments. Earth Planet. Sci. Lett. 1987, 86, 389–400. [Google Scholar] [CrossRef]
- Meftahi, A.; Samyn, P.; Geravand, S.A.; Khajavi, R.; Alibkhshi, S.; Bechelany, M.; Barhoum, A. Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: Formulations, regulations, and emerging applications. Carbohydr. Polym. 2022, 278, 118956. [Google Scholar] [CrossRef]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant Celluloses, Hemicelluloses, Lignins, and Volatile Oils for the Synthesis of Nanoparticles and Nanostructured Materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef] [PubMed]
- Sali, W.; Patoli, D.; Pais de Barros, J.-P.; Labbé, J.; Deckert, V.; Duhéron, V.; Le Guern, N.; Blache, D.; Chaumont, D.; Lesniewska, E.; et al. Polysaccharide Chain Length of Lipopolysaccharides from Salmonella Minnesota Is a Determinant of Aggregate Stability, Plasma Residence Time and Proinflammatory Propensity in vivo. Front. Microbiol. 2019, 10, 1774. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Alvarado, R.B.; Nazarenko, Y.; Ariya, P.A. Snow-borne nanosized particles: Abundance, distribution, composition, and significance in ice nucleation processes. J. Geophys. Res. Atmos. 2015, 120, 11760–11774. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B. Biomimetics: Lessons from naturean overview. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1445–1486. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Kolle, S.; Weaver, J.C.; Ortiz, C.; Aizenberg, J.; Kolle, M. A highly conspicuous mineralized composite photonic architecture in the translucent shell of the blue-rayed limpet. Nat. Commun. 2015, 6, 6322. [Google Scholar] [CrossRef] [Green Version]
- Seehra, M.; Bristow, A. (Eds.) Noble and Precious Metals: Properties, Nanoscale Effects and Applications; IntechOpen: London, UK, 2018. [Google Scholar]
- Zhang, C.; Sun, L.D.; Yan, C.H. Noble metal plasmonic nanostructure related chromisms. Inorg. Chem. Front. 2016, 3, 203–217. [Google Scholar] [CrossRef]
- Forehand, C.J. Bioinspired Study on the Mechanical Performance of Helicoidal Fiber Structures. Master’s Thesis, Faculty of the Graduate College of The University of Vermont, Burlington, VT, USA, 2015. [Google Scholar]
- Floody, M.C.; Theng, B.K.G.; Reyes, P.; Mora, M.L. Natural nanoclays: Applications and future trends—A Chilean perspective. Clay Miner. 2009, 44, 161–176. [Google Scholar] [CrossRef]
- Hochella, M.F.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, Mineral Nanoparticles, and Earth Systems. Science 2008, 319, 1631–1635. [Google Scholar] [CrossRef] [Green Version]
- Souto Filho, S.N.; Alves, M.C.; Monreal, C.M.; Bonini, C.D.S.B. Nanoparticles and nanostructure morphology of a Red Latosol in rehabilitation. Rev. Bras. Eng. Agrícola Ambient. 2017, 21, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Liu, Y.; Kihm, K.D.; Hill, E.; Taylor, L.A. Submicron Particle Size Distribution of Apollo 11 Lunar Dust. In Earth & Space 2006: Engineering, Construction, and Operations in Challenging Environment; American Society of Civil Engineers: Reston, VA, USA, 2006; Volume 2006, pp. 1–6. [Google Scholar] [CrossRef]
- Krisanova, N.; Kasatkina, L.; Sivko, R.; Borysov, A.; Nazarova, A.; Slenzka, K.; Borisova, T. Neurotoxic Potential of Lunar and Martian Dust: Influence on Em, Proton Gradient, Active Transport, and Binding of Glutamate in Rat Brain Nerve Terminals. Astrobiology 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chastain, B.K.; Kral, T.A. Zero-valent iron on Mars: An alternative energy source for methanogens. Icarus 2010, 208, 198–201. [Google Scholar] [CrossRef]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holbrook, W.S.; Marcon, V.; Bacon, A.R.; Brantley, S.L.; Carr, B.J.; Flinchum, B.A.; Richter, D.D.; Riebe, C.S. Links between physical and chemical weathering inferred from a 65-m-deep borehole through Earth’s critical zone. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, J.; Schulze, C.; Marxer, E.E.J.; Schaefer, U.F.; Wohlleben, W.; Bakowsky, U.; Lehr, C.M. Atomic force microscopy and analytical ultracentrifugation for probing nanomaterial protein interactions. ACS Nano 2012, 6, 4603–4614. [Google Scholar] [CrossRef] [PubMed]
- Mecham, R.P.; Birk, D.E. Extracellular Matrix Assembly and Structure; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Sinden, R.R. DNA twists and flips. Nature 2005, 437, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Nanostructured scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101A, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Tadano, S.; Giri, B. X-ray diffraction as a promising tool to characterize bone nanocomposites. Sci. Technol. Adv. Mater. 2012, 12, 064708. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 1–33. [Google Scholar] [CrossRef]
- Suzan-Monti, M.; La Scola, B.; Raoult, D. Genomic and evolutionary aspects of Mimivirus. Virus Res. 2006, 117, 145–155. [Google Scholar] [CrossRef]
- Desjardins, C.A. Unusual Viral Genomes: Mimivirus and the Polydnaviruses. Parasit. Viruses Symbionts Pathog. 2012, 115–125. [Google Scholar] [CrossRef]
- Shah, M.; Badwaik, V.; Kherde, Y.; Waghwani, H.K.; Modi, T.; Aguilar, Z.P. Gold Nanoparticles: Various Methods of Synthesis and Antibacterial Applications. Front. Biosci. 2014, 19, 1320–1344. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef] [Green Version]
- Barhoum, A.; Li, H.; Chen, M.; Cheng, L.; Yang, W.; Dufresne, A. Emerging Applications of Cellulose Nanofibers. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 1131–1156. [Google Scholar]
- Rühs, P.A.; Storz, F.; López Gómez, Y.A.; Haug, M.; Fischer, P. 3D bacterial cellulose biofilms formed by foam templating. Npj Biofilms Microbiomes 2018, 4, 21. [Google Scholar] [CrossRef]
- Huber, J.T.; Noyes, J.S. A new genus and species of fairyfly, Tinkerbella nana (Hymenoptera, Mymaridae), with comments on its sister genus Kikiki, and discussion on small size limits in arthropods. J. Hymenopt. Res. 2013, 32, 17–44. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, T.B.H.; Houghtaling, J.; Wilts, B.D.; Mayer, M. It’s Not a Bug, It’s a Feature: Functional Materials in Insects. Adv. Mater. 2018, 30, 1705322. [Google Scholar] [CrossRef] [Green Version]
- Wootton, R.J. Functional Morphology of Insect Wings. Annu. Rev. Entomol. 2003, 37, 113–140. [Google Scholar] [CrossRef]
- Yoshioka, S.; Kinoshita, S. Direct determination of the refractive index of natural multilayer systems. Phys. Rev. E 2011, 83, 051917. [Google Scholar] [CrossRef]
- Nguyen, S.; Webb, H.; Mahon, P.; Crawford, R.; Ivanova, E. Natural Insect and Plant Micro-/Nanostructsured Surfaces: An Excellent Selection of Valuable Templates with Superhydrophobic and Self-Cleaning Properties. Molecules 2014, 19, 13614–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, D.; Hong, J.; Ko, J.H.; Lee, Y.J.; Park, H.C.; Byun, B.K.; Lukes, J.R. Wetting Characteristics of Insect Wing Surfaces. J. Bionic Eng. 2009, 6, 63–70. [Google Scholar] [CrossRef]
- Watson, G.S.; Cribb, B.W.; Watson, J.A. Contrasting micro/nano architecture on termite wings: Two divergent strategies for optimising success of colonisation flights. PLoS ONE 2011, 6, e024368. [Google Scholar] [CrossRef] [Green Version]
- Eliason, C.M.; Bitton, P.P.; Shawkey, M.D. How hollow melanosomes affect iridescent colour production in birds. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131505. [Google Scholar] [CrossRef] [Green Version]
- Saranathan, V.; Forster, J.D.; Noh, H.; Liew, S.F.; Mochrie, S.G.J.; Cao, H.; Dufresne, E.R.; Prum, R.O. Structure and optical function of amorphous photonic nanostructures from avian feather barbs: A comparative small angle X-ray scattering (SAXS) analysis of 230 bird species. J. R. Soc. Interface 2012, 9, 2563–2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Biswas, S.K.; Han, J.; Tanpichai, S.; Li, M.C.; Chen, C.; Zhu, S.; Das, A.K.; Yano, H. Surface and Interface Engineering for Nanocellulosic Advanced Materials. Adv. Mater. 2021, 33, 2002264. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Jakes, J.E.; Zelinka, S.L.; Hunt, C.G.; Ciesielski, P.; Frihart, C.R.; Yelle, D.; Passarini, L.; Gleber, S.C.; Vine, D.; Vogt, S. Measurement of moisture-dependent ion diffusion constants in wood cell wall layers using time-lapse micro X-ray fluorescence microscopy. Sci. Rep. 2010, 10, 9919. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Arzt, E.; Gorb, S.; Spolenak, R. From micro to nano contacts in biological attachment devices. Proc. Natl. Acad. Sci. USA 2003, 100, 10603–10606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lespes, G.; Faucher, S.; Slaveykova, V.I. Natural Nanoparticles, Anthropogenic Nanoparticles, Where Is the Frontier? Front. Environ. Sci. 2020, 8, 71. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Hii, Y.S.; Chan, Y.S. Biosynthesized Metal Nanoparticles in Bioremediation. In Rhizomicrobiome Dynamics in Bioremediation; CRC Press: Boca Raton, FL, USA, 2021; pp. 126–161. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered nanomaterials for plant growth and development: A perspective analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef]
- Singh, N.; Bhuker, A.; Jeevanadam, J. Effects of metal nanoparticle-mediated treatment on seed quality parameters of different crops. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021, 394, 1067–1089. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Pan, S.; Harini, A.; Danquah, M.K. Challenges in the Risk Assessment of Nanomaterial Toxicity Towards Microbes. Interfaces Between Nanomater. Microbes 2021, 58–93. [Google Scholar] [CrossRef]
- Gupta, S.; Shanker, R.; Dhawan, A.; Kumar, A.; Gupta, G.S.; Shanker, R.; Kumar, A.; Dhawan, A. Impact of Nanomaterials on the Aquatic Food Chain. Sustain. Agric. Rev. 2017, 26, 309–333. [Google Scholar] [CrossRef]
- Rastogi, A.; Singh, P.; Haraz, F.A.; Barhoum, A. Chapter 19—Biological synthesis of nanoparticles: An environmentally benign approach. In Micro and Nano Technologies; Barhoum, A., Hamdy Makhlouf, A.S.B.T.-F., Hamdy Makhlouf, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 571–604. ISBN 978-0-323-51255-8. [Google Scholar]
- Donaldson, K.; Tran, L.; Jimenez, L.A.; Duffin, R.; Newby, D.E.; Mills, N.; MacNee, W.; Stone, V. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Part. Fibre Toxicol. 2005, 2, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musee, N. Nanowastes and the environment: Potential new waste management paradigm. Environ. Int. 2011, 37, 112–128. [Google Scholar] [CrossRef]
- Hou, L.; Li, K.; Ding, Y.; Li, Y.; Chen, J.; Wu, X.; Li, X. Removal of silver nanoparticles in simulated wastewater treatment processes and its impact on COD and NH4 reduction. Chemosphere 2012, 87, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Blaser, S.A.; Scheringer, M.; MacLeod, M.; Hungerbühler, K. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nanofunctionalized plastics and textiles. Sci. Total Environ. 2008, 390, 396–409. [Google Scholar] [CrossRef]
- Blinova, I.; Ivask, A.; Heinlaan, M.; Mortimer, M.; Kahru, A. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ. Pollut. 2010, 158, 41–47. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotechnology 2011, 5, 43–54. [Google Scholar] [CrossRef]
- Ellegaard-Jensen, L.; Jensen, K.A.; Johansen, A. Nano-silver induces dose-response effects on the nematode Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2012, 80, 216–223. [Google Scholar] [CrossRef]
- Said, M.M.; Rehan, M.; El-Sheikh, S.M.; Zahran, M.K.; Abdel-Aziz, M.S.; Bechelany, M.; Barhoum, A. Multifunctional Hydroxyapatite/Silver Nanoparticles/Cotton Gauze for Antimicrobial and Biomedical Applications. Nanomaterials 2021, 11, 429. [Google Scholar] [CrossRef]
- Hussain, S.M.; Javorina, A.K.; Schrand, A.M.; Duhart, H.M.H.M.; Ali, S.F.; Schlager, J.J. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol. Sci. 2006, 92, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Warheit, D.B.; Webb, T.R.; Colvin, V.L.; Reed, K.L.; Sayes, C.M. Pulmonary bioassay studies with nanoscale and fine-quartz particles in rats: Toxicity is not dependent upon particle size but on surface characteristics. Toxicol. Sci. 2007, 95, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, C.C.; Hsiao, H.Y.; Hong, Q.S.; Chen, C.H.; Peng, Y.W.; Chen, H.W.; Yang, P.C. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008, 8, 437–445. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanoparticle Res. 2010, 1645–1654. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to escherichia Coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Braida, W.; Christodoulatos, C.; Wazne, M.; O’Connor, G. Nano-aluminum: Transport through sand columns and environmental effects on plants and soil communities. Environ. Res. 2008, 106, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef]
- Lippmann, M. Effects of fiber characteristics on lung deposition, retention, and disease. In Proceedings of the Environmental Health Perspectives; US Department of Health and Human Services: Washington, DC, USA, 1990; Volume 88, pp. 311–317. [Google Scholar]
- Gurr, J.R.; Wang, A.S.S.; Chen, C.H.; Jan, K.Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Johnston, C.J.; Finkelstein, J.N.; Mercer, P.; Corson, N.; Gelein, R.; Oberdörster, G. Pulmonary effects induced by ultrafine PTFE particles. Toxicol. Appl. Pharmacol. 2000, 168, 208–215. [Google Scholar] [CrossRef]
- Vetreno, R.P.; Crews, F.T. Current hypotheses on the mechanisms of alcoholism. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 125, pp. 477–497. [Google Scholar]
- D’Silva, J.; Van Calster, G. Taking temperature—A review of European union regulation in nanomedicine. Eur. J. Health Law 2009, 16, 249–269. [Google Scholar] [CrossRef]
- Marchant, G.E.; Sylvester, D.J.; Abbott, K.W.; Danforth, T.L. International harmonization of regulation of nanomedicine. Stud. Ethics. Law. Technol. 2009, 3. [Google Scholar] [CrossRef]
- Ajazzuddin, M.; Jeswani, G.; Jha, A. Nanocosmetics: Past, Present and Future Trends. Recent Pat. Nanomed. 2015, 5, 3–11. [Google Scholar] [CrossRef]
- Johnson, V.R. Nanotechnology, environmental risks, and regulatory options. Penn St. L. Rev. 2016, 121, 471. [Google Scholar]
- Thomas, T.; Thomas, K.; Sadrieh, N.; Savage, N.; Adair, P.; Bronaugh, R. Research strategies for safety evaluation of nanomaterials, part VII: Evaluating consumer exposure to nanoscale materials. Toxicol. Sci. 2006, 91, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Wiedermann, C.J.; Eisendle, K. Comparison of hydroxyethyl starch regulatory summaries from the Food and Drug Administration and the European Medicines Agency. J. Pharm. Policy Pract. 2017, 10, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, R. Societal Age Stereotypes in the U.S. and U.K. from a Media Database of 1.1 Billion Words. Int. J. Environ. Res. Public Health 2021, 18, 8822. [Google Scholar] [CrossRef]
- Slocum, J.M.; Letendre, M. Voice of Experience Recent Developments in Human Research Protection in Canada Introduction. VOE J. Res. Adm. 2011, XLII, 131. [Google Scholar]
- Guzmán, K.A.D.; Taylor, M.R.; Banfield, J.F. Environmental risks of nanotechnology: National nanotechnology initiative funding, 2000–2004. Environ. Sci. Technol. 2006, 40, 1401–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]























| Organization | Term | Definition | Ref. |
|---|---|---|---|
| British Standards Institution | Nanoscale | Size range approximately from 1 to 100 nm. | [84] |
| Technical Committees of the International Standardization Organization | Nanotechnology | Scientific information used to manipulate and control nano-matter (100 nm) to exploit its size- and structure-specific features and properties that are different from those of individual atoms/molecules or bulk materials. | [85] |
| European Patent Office | Nanotechnology | Describes entities with a controlled geometrical size <100 nm of at least one functional component in one or more dimensions that can lead to size-dependent physical, chemical, or biological effects. | [86] |
| American National Standards Institute—Nanotechnology Standards Panel | Nanotechnology | Understanding and controlling matter of ~1–100 nm in size, where unique phenomena allow new applications. Nanotechnology comprises nanoscale science, engineering and technology, in which nanomaterials are imaged, measured, modeled and manipulated. | [87] |
| British Standards Institution | Nanoscience | Study of nano-scaled matter to understand their size- and structure-dependent features and to compare individual atoms or molecules or bulk material related differences. | [84] |
| European Union Scientific Committee on Consumers Products | Nanomaterials | Materials that have one or more external dimensions, or an internal structure in the nanoscale, and may display novel features compared with the same material but not in the nanoscale. Here, nanoscale means that ≥1 dimension(s) is ≤100 nm. | [88] |
| European Commission: Cosmetic Products Regulation | Nanomaterials | Insoluble or bio-persistent and purposely produced material with one or more external dimensions, or an internal structure in the 1–100 nm size. | [89,90] |
| American Chemistry Council | Engineered nanomaterial | Any purposely produced material with a size in 1, 2, or 3 dimensions between 1 and 100 nanometers. However, (i) materials without novel/unique/new features compared with the bulk materials; (ii) materials soluble in water or biologically relevant solvents at the molecular level, and (iii) micelles and single-polymer molecules are excluded. | [91] |
| European Commission | Nanomaterials | Any natural, incidental, or manufactured material that includes unbound, aggregated or agglomerated particles of which ≥50% are in the 1–100 nm size range. Based on specific environmental, health, safety concerns, or competitiveness issues, this threshold may be replaced by a threshold between 1 and 50%. Fullerenes, graphene flakes, and single-wall carbon nanotubes with one or more external dimensions <1 nm also are classified as nanomaterials. | [92] |
| European Commission for novel foods (amending Regulation No258/97), under discussion | Nanomaterials | Any purposely produced material with ≥1 dimension ≤100 nm or composed of discrete functional parts (internally or at the surface), many of which have ≥1 dimension ≤100 nm. This comprises also structures, agglomerates, and aggregates with a size >100 nm but with nanoscale-specific properties. | [92] |
| British Standards Institution | Nano-object | Material with one or more peripheral nanoscale dimensions. | [93] |
| British Standards Institution | Nanoparticle | Nano-object with three external nanoscale dimensions. The terms nanorod, nanoplate, nanosheets are used at the place of nanoparticle when the nanoobject longest and shortest axis lengths are different. | [94] |
| British Standards Institution | Nanofiber | Nanomaterial with two similar exterior nanoscale dimensions and a third larger dimension. | [95] |
| British Standards Institution | Nanocomposite | Multiphase structure with at least one phase in the nanoscale dimension. | [96] |
| British Standards Institution | Nanostructure | Structure of interconnected constituent parts in the nanoscale region. | [1] |
| British Standards Institution | Nanostructured materials | Materials with internal or surface nanostructures. | [97] |
| Zeta Potential (mV) | Dispersion Stability |
|---|---|
| 0–5 mV | Instable |
| 10–30 mV | Incipient instability |
| 30–40 mV | Moderate stability |
| 40–60 mV | High stability |
| >60 mV | Excellent stability |
| Properties | Example | Ref. |
|---|---|---|
| Catalytic activity | The catalytic activity of NPs is influenced by their size and shape. The catalyst activity is inversely proportional to the required catalytic activation energy. The catalytic activation energy of TiO2, SnO2 and CeO2 NPs decreases with the increase of their size. However, catalytic activation energy becomes almost size- independent when the NP size is >10–15 nm. TiO2, SnO2 and CeO2 NPs with a tetrahedral shape display the lowest catalytic activation energy, and therefore are more efficient catalysts. | [198] |
| Electrical properties | Electrical properties (e.g., conductivity or resistivity) change from the microscale to the nanoscale. The conductivity of a bulk carbon material is not influenced by its size (diameter) and twisting. Conversely, the conductivity of CNTs is modified by the cross-section area changes and upon application of shear forces (twisting). In addition, electrical conductivity of a MWCNTs is different from that of SWCNTs with the same dimensions. | [199] |
| Magnetic properties | The magnetic characteristics of NPs are modulated by so-called finite-size and surface effects. Finite-size effects are induced by the quantum confinement o electrons. Surface effects can be caused by the symmetry breaking of the crystal structure at the boundary of each particle, but also by the different chemical and magnetic structures of the NP core and outer shell. | [200] |
| Optical properties | The spectral shift of optical absorption and fluorescence properties increase the quantum efficiency of semiconductor crystals. For example, 20-nm gold (Au), platinum (Pt), silver (Ag), and palladium (Pd) NPs display a typical red wine, yellowish gray, black, and dark black color, respectively. | [201,202] |
| Steric features | Hollow spheres show higher selectivity for specific drug transport and controlled release. Nano drug-delivery systems (DDS) are developed to increase the drug efficiency and reduce its toxicity of loaded drugs, but only few are currently used in the clinic. | [203,204,205], |
| Biological features | Higher permeability through biological barriers (e.g., cell membranes, blood-brain barrier), and higher biocompatibility. NP ability to organize around proteins is mainly influenced by their size, curvature, shape and surface characteristics (charge, functionalization, and free energy). | [206,207] |
| Occurrence | Nanostructures | Thickness/Diameter (nm) | Ref. |
|---|---|---|---|
| Human body | DNA | 2–2.5 nm | [210] |
| Enzymes | 3–7 nm | [211] | |
| Antibodies | 10–15 nm | [212] | |
| Bone Collagen fibrils | 60–70 nm | [213] | |
| Mitochondria | 1000 nm | [214] | |
| Birds | Feathers (peacock) | <100 nm | [215] |
| Feathers (scarlet macaw) | <100 nm | [216] | |
| Feathers (toucan) | <100 nm | [217] | |
| Feathers (blue jay) | <100 nm | [218] | |
| Other vertebrates and insects | Panther chameleon skin | 10–100 nm | [219] |
| Poison dart frog skin | 10–80 nm | [220] | |
| Moth eyes | ~100 nm | [221] | |
| Butterfly wings | ~100 nm | [222] | |
| Opal weevil (beetle) | 2–10 nm (Photonic Nanocrystals) | [223] | |
| Microorganisms | Magnetotactic bacteria | Bacteria produces magnetite nanocrystals ~50–100 nm | [224] |
| Bacterial Cellulose | Bacteria produces celluloses nanofibers ~20–40 nm diameter | [225,226] | |
| Bacteria | 0.6–5000 nm | [227] | |
| Virus | 50–150 nm | [228] | |
| Fungi | Setae with a spatula of 50–100 nm in size | [229] | |
| Aquatic ecosystems | Blue-rayed limpet | Shell containing 50–100 nm particles | [230] |
| Clownfish | 10–100 nm | [231] | |
| Blue-ringed octopus | 10–100 nm | [232] | |
| Lobster eggs | 500–600 nm | [233] | |
| Soil | Clays | 3.5–5 nm | [234] |
| Manganese hydrous oxide nano-minerals | 3–5 nm | [235] | |
| Ferric iron hematite crystals | 5–6 nm | [235] | |
| Red latosol topsoil | 200 nm | [236] | |
| Space | Lunar dust | 500 nm | [237] |
| Mars dust particles | 50–200 | [238] | |
| Mars soil | 30–100 nm | [239] |
| Environment Type | Environmental Risks | Ref. |
|---|---|---|
| Air | Nanoparticles can be formed in urban areas, several combustion sources (engines, biomass burning, power generation plants) are directly emitting carbonous nanoparticles to the atmosphere. They classify as nanosized pollutants in industrialized areas. | [277] |
| Soil | Metal nanoparticles from the utilized biomass can be transferred to soil by nanoparticles sorption. Therefore, the high Ag concentration in the resulting biomass might restrict its agricultural use; the concentration would act as an inhibitor of bacterial growth (including the beneficial microorganisms present in the soil, such as nitrogen-fixing bacteria). | [278,279] |
| Water | Antimicrobial metal and metal oxide nanoparticles have a toxic effect in model organisms (e.g., nematodes, zebrafish embryos) suggesting that cumulative exposure to silver nanoparticles might significantly affect the ecological balance of aquatic environments. It is crucial to carefully assess the possible eco-toxicity when large amounts of manufactured oxide nanoparticles are released in natural waters. | [280,281,282,283,284,285] |
| Humans | Exposure to carbon nanomaterials and silica nanoparticles can cause lung inflammation, granuloma, and fibrosis. Carbon, Ag, and Au nanomaterials can reach also other organs (e.g., central nervous system). Quantum dots, carbon, and TiO2 NPs can go through the skin barrier. MnO2, TiO2, and carbon nanoparticles may enter the brain via olfactory neurons in the nasal epithelium. TiO2, Al2O3, carbon black, Co, and Ni NPs might be more toxic than micro-particles. | [286,287,288] |
| Microorganisms | Toxicities associated with nanoparticles in microorganisms are mainly related to their nanosize effect that causes membrane disorganization, generation of reactive oxygen species (ROS), and in some cases, oxidative DNA damage. | [289,290] |
| Plants | Nanoparticles may also adhere to the roots of the plants and cause physical or chemical toxicity to plants. Some nanoparticles were observed to be nontoxic to plant (SiO2 NPs), but some studies observed the toxic effect due to decrees in pH of the media after addition of these nanoparticles. The Zn- and Al-containing nanoparticles negatively affect germination and root growth of agriculturally relevant plant species. | [291,292] |
| Factor | Nanomaterial Toxicity Effect | Ref. |
|---|---|---|
| Dose and exposure duration | Nanoparticle’s concentration in the medium multiplied by the exposure duration directly determines the number of nanoparticles in the body cells. | [207,124] |
| Aggregation and concentration | Data on nanoparticles toxicity as a function of their concentration are not clear-cut. Higher nanoparticles concentrations favor their aggregation. As the size of nanoparticles aggregates is often in the micrometer range, aggregated nanoparticles may not easily enter cells, then their toxicity is decreased/abolished. | [1] |
| Nanoparticle size | Nanoparticles’ size influences toxicity. For instance, cell penetration and toxicity are higher for small Ag NPs (~10 nm) than for Ag+ ions and larger Ag NPs (20–100 nm). | [293] |
| Nanoparticle morphology | Nanoparticle’s toxicity varies in function of their aspect ratio. For instance, asbestos fibers of 10 µm in length may cause lung cancer, fibers between 5 and 10 µm may cause mesothelioma, and shorter fibers (2 µm) may cause asbestosis. | [294] |
| Surface area | Nanoparticle’s toxicity progressively increases with smaller size and larger surface area. Moreover, nanoparticles and microparticles used at the same mass dose have different effects in human cells. | [1] |
| Crystal structure | Nanoparticles’ crystal structure influences the cellular uptake, oxidative mechanisms, and subcellular localization. For instance, the two TiO2 NPs polymorphs (rutile and anatase crystal structures) display different toxic effects. In the dark, rutile TiO2 NPs (200 nm) cause DNA damage by oxidation and this manages not caused by anatase nanoparticles (200 nm). | [2] |
| Surface functionalization | Nanoparticles’ surface functionalization (hydrophilicity and surface charge) strongly influences their translocation and oxidation and DNA damage. | [266] |
| Pre-exposure | The phagocytic activity of cells may be promoted by shorter exposure times or by pre-exposure to NPs at lower doses (to somehow adapt the human body to that nanoparticle). | [268] |
| Organization | Risks and Regulations | Ref. |
|---|---|---|
| Scientific Committee on Consumer Products (SCCS)—European Union | Medical standards related to ethics, environmental safety, and medical governance were revised to cover biomedical applications of nanomaterials. Currently, the most influential regulatory bodies and stricter guidelines are in the USA and EU. The European Commission (EC) published legislation and technical recommendations on nanomaterials to guarantee conformity within all EU countries and among the different sectors. As nanomaterials meet the European Registration, Evaluation, Authorization, and Restriction of Chemical (REACH) and the European Classification and Labelling of Chemicals substance definitions, their regulations also apply to nanomaterials. | [300,301] |
| Scientific Committee on Emerging and Newly-Identified Health Risks (SCENIHR)—European Union | The mission of SCENIHR includes assessing the risks of nanomaterials. According to the Directive 76/768/EEC of 2013, which replaced the EU cosmetics regulation 1223/2009, a nanomaterial is “an insoluble or bio-persistent and internationally manufactured material with one or more external dimensions, or an internal structure in the range of 1 to 100 nm which includes man-made fullerene, single-walled carbon nanotubes, and graphene flakes”. Moreover, several bodies in charge of cosmetics safety and regulations, such as the US Federal Food, Drug, and Cosmetic Act, Personal Care Products Council and Voluntary Cosmetic Registration Program, the EU Cosmetics Product Notification Portal, REACH and Scientific Committee on Consumer Safety, and the International Cooperation on Cosmetic Regulation highlighted the issue of the toxicity of NM contained in cosmetics. | [302,303] |
| Food and Drug Administration (FDA), Environmental Protection Agency (EPA) in USA | These regulatory agencies have set up protocols to monitor and manage the risks of nanomaterials and nanoproducts. Since 2006 the FDA identified nanomaterials sources, assessing the environmental impact and risks for humans, fauna, and flora, and developing protocols of nanomaterials to avoid or limit these risks. | [304] |
| European Medicines Agency (EMEA), and US Food and Drug Administration (FDA) | EMEA and FDA contribute to the regulation of the biomedical applications of hazardous nanomaterials. Moreover, a league of US and international advocacy groups published the book “Principles for the oversight of nanotechnologies and Nanomaterials” endorsed by 70 groups in six continents. This book advocates a thorough evaluation of all nanomaterials-derived products. It includes the need for specific NM regulations based on the precautionary principle of health and safety regulations. These principles approach for consumers and workers, transparency, public participation, and environmental protection. Likewise, the Nanomaterials Policy Recommendations report describes how to avoid or limit the risk of nanomaterials in food industries. It also suggests that industries should put in place a detailed public policy for nanomaterials use, to make public the nanomaterials safety analyses, set up supplier standards, indicate the presence of nanomaterials <500 nm in size, and implement hazard control strategies to avoid nanomaterials exposure. | [305] |
| UK Soil Association, Biological Farmers of Australia (BFA), Canada General Standards Board (CGSB) | These organic food/farming bodies have already forbidden the use of man-made nanomaterials in food. Scientists and industry should be aware of the existing regulation and legislation before nanomaterials production. It is acknowledged that nanomaterials are not intrinsically dangerous and that many nanomaterials are non-toxic, and others have beneficial health effects. However, future risk assessments will determine whether or not nanomaterials and derivatives are dangerous and whether additional actions are required. | [306,307] |
| National Nanotechnology Initiative (NNI) in USA | Nanoscale Science, Engineering, and Technology committee created the NNI to coordinate US government funding for nanotechnology research and to support the nanotechnology industry. | [1] |
| National Science Foundation (NSF) in USA | NSF promotes discussions on the nanotechnology risks by organizing workshops, reports, conferences, and publications. | [1] |
| Action Group on Erosion, Technology, and Concentration (ETC Group)—Canada | This group is at the origin of a call for a moratorium on the experimental use and commercial development of synthetic nanomaterials based on the precautionary principle, the limited knowledge on nanotechnology risks, and the absence of best practices for nanomaterials handling and use. | [1] |
| Chemical Industry Vision 2020 Technology Partnership | This partnership defined an R&D roadmap that focuses on (i) assessing the nanomaterials risks for human health and the environment, (ii) analyzing the risk of exposure to nanomaterials, and (iii) defining guidelines to handle nanomaterials. | [1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; S. Abdalla, M.; Bechelany, M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials 2022, 12, 177. https://doi.org/10.3390/nano12020177
Barhoum A, García-Betancourt ML, Jeevanandam J, Hussien EA, Mekkawy SA, Mostafa M, Omran MM, S. Abdalla M, Bechelany M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials. 2022; 12(2):177. https://doi.org/10.3390/nano12020177
Chicago/Turabian StyleBarhoum, Ahmed, María Luisa García-Betancourt, Jaison Jeevanandam, Eman A. Hussien, Sara A. Mekkawy, Menna Mostafa, Mohamed M. Omran, Mohga S. Abdalla, and Mikhael Bechelany. 2022. "Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations" Nanomaterials 12, no. 2: 177. https://doi.org/10.3390/nano12020177