Possible Synergies of Nanomaterial-Assisted Tissue Regeneration in Plasma Medicine: Mechanisms and Safety Concerns
Abstract
:1. Introduction
2. Nanomaterials in Regenerative Medicine and Tissue Engineering
3. Plasma Medicine for Regenerative Medicine and Tissue Engineering
3.1. The Basic Working Principle behind Biomedical Plasma Treatment
- An external electric field accelerates the electrons, resulting in ionization, excitation and dissociation of the feed gas (usually an inert gas such as argon, helium, or a mixture of different gases such as ambient air, oxygen, nitrogen) via electron impact, resulting in ions, excited species, and radicals or smaller molecules/atoms, respectively.
- During the plasma discharge, highly charged atoms, molecules, and free electrons subsequently interact with other particles (ambient air, liquids, surfaces), and they can generate secondary and tertiary reactive species.
- The corresponding excitation and depletion processes and the charge transport eventually lead to the formation of UV, visible light, and IR, which gives the plasma its characteristic color.
- the complete inactivation of multidrug-resistant microbes;
- stimulation of tissue regeneration by means of cell proliferation and angiogenesis at a lower dose of CAP treatment; and
- triggering programmed cell death, primarily in cancer cells, often at higher CAP intensity and treatment time.
3.2. Effect of the Plasma-Induced Redox Chemistry
3.3. Effect of the Plasma-Induced Electric Fields
| Endogenous Electric Field | Natural Biological Phenomenon | Reference |
| 0.005–0.07 V/cm | Neuronal excitability | [188,189,190,191,192,193,194] |
| 0.21–10 V/cm | Embryonic transneural tube potential for ion transport | [195,196,197] |
| 0.4–2 V/cm | Laterally oriented field during wound healing | [183,198,199,200,201] |
| ~2 V/cm | Skin battery of intact skin | [180] |
| Exogenous Electric Field | Effect on Biological Matter | Reference |
| 0.07–0.09 V/cm | Cell reorientation | [202] |
| 0.03–10 V/cm | Directional migration of different cell types | [182,184,203,204,205,206] |
| 0.16–4.4 V/cm | Directional migration of neural stem or progenitor cells | [207,208,209,210,211,212] |
| 0.1–1.5 V/cm | Directional nerve growth | [183,213,214] |
| 0.28–2.2 V/cm | Neuron orientation in vitro | [215] |
| 1–2.5 V/cm | Lens and corneal epithelial cell reorientation | [203,216,217] |
| 0.17 V/cm | Keratinocyte differentiation | [218] |
| 0.3–3 V/cm | Neuronal differentiation | [219,220,221,222] |
| 1 V/cm | Cell proliferation in wound healing | [223] |
| 2 V/cm | Enhanced osteoblast proliferation for bone tissue regeneration | [224] |
| 3–4 V/cm | Depressed osteoblast proliferation | [224] |
| 1–3 V/cm | Iontophoresis | [225,226,227] |
| ~1–20 V/cm | Pulsed electric field for electro-endocytosis | [151,162,228,229] |
| 400–500 V/cm | Pulsed electric field for electroporation of mammalian cells | [230,231] |
| ~102–104 V/cm | Pulsed electric field for electroporation of different cell types | [151] |
| ~103 V/cm | Pulsed electric field for cell electrofusion | [162] |
| 1 V/cm | Directional protein migration | [183] |
| 2 V/cm | Enhanced bone protein production | [224] |
| ≥10 V/cm | Protein crystallization | [232] |
| ~104–5 × 104 V/cm | Pulsed electric field for enzyme deactivation | [233,234,235] |
3.4. Effect of the Plasma-Induced Magnetic Fields
4. The Search for Synergies between Plasma and Nanomedicine
4.1. Enhancing the Strengths and Overcoming the Limitations
4.2. What Can We Learn from Cancer Research?
| Nanoparticles | Plasma Entity | Finding | Type of Cancer |
|---|---|---|---|
| Gold-NPs | Helium Plasma jet | Activation of intracellular ROS contents, cell cycle arrest, and intracellular anti-oxidant machinery leads to early apoptosis | myelomonocytic lymphoma [285] |
| Plasma jet | Activation of intracellular ROS levels leads to cancer cell death | Glioblastoma [104] | |
| Cell death due to enhancement of intracellular RONS level and uptake of NPs | Brain tumor [102] | ||
| Plasma jet | Cell death due to nuclear condensation and DNA fragmentation | Colorectal cancer [286] | |
| Gas Plasma | Decrease in cell proliferation and migration with induction of cancer cell death | Breast cancer [287] | |
| Production of intracellular RONS causes significant lipid peroxidation due to an increase in the uptake of gold-NPs through endocytosis | Brain tumor [104] | ||
| Anti-EGF receptor gold-NPs | Air plasma | Necrotic cell death | Skin and oral cancer [144] |
| EGF-conjugated gold-NPs | Selective apoptosis of cells having EGF receptor-mediated endocytosis | EGF receptor-expressing lung carcinoma cell [284] | |
| EGF conjugated gold-NPs | DBD plasma | Increase the apoptotic response | Lung cancer [284] |
| Focal Adhesion Kinase antibody conjugated gold-NPs | Gap 1 (G1) phase cell cycle arrest leads to apoptosis | Skin cancer [109] | |
| Gold-NPs | Stimulates clathrin-dependent endocytosis | Glioblastoma [144] | |
| Polyethylene glycol (PEG) gold-NPs | Plasma jet | Singlet oxygen formation and formation of gold-PEG bond | Skin and oral cancer [288] |
| Fluorouracil (5-FU)loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles | induced down-regulation of metastasis-related gene expression | breast tumors [282] | |
| Curcumin loaded on triphosphate chitosan NPs | Gap 2 phase/mitosis (G2/M) cell cycle arrest, upregulation of tumor suppressor markers | Breast cancer [289] | |
| Iron-NPs | Tumor size reduction due to extensive necrosis | Lung Cancer [283] | |
| PLGA-magnetic iron oxide | Triggering apoptotic process with DNA fragmentation | Lung cancer [112] | |
| Platinum-NPs | Apoptosis due to cell cycle arrest, DNA fragmentation and augmentation of Ca2+ labels | Lymphoma [290] | |
| Fluorouracil loaded PLGA NPs | Cytotoxic effects, metastatic gene expression reduction with the uptake of NPs | Breast cancer [282] |
4.3. Exploring the Boundaries with Biotechnology
4.4. In Situ Plasma Modification of Nanomaterials
4.5. Redox-Related Synergies
- In line with Section 4.4, plasma-generated RONS can modify and consume the nanomaterial surface at a controllable rate. This provides a means to vary the release rate of NP matter, to regulate the location where such release occurs and/or to completely degrade a nanomaterial in situ, all very useful abilities to restrict short- and long-term toxicities.
- Reversely, CAP is, in theory, able to supply or resupply nanomaterials with RONS, for instance by an adhesion or deposition process at their surface. In other words, nanomaterials can function as RONS batteries that can be recharged by means of plasma treatment. This enables an interesting flexibility, since such nanomaterials may be neutral to the redox chemistry in their completely discharged state, and charge selectively or unselectively with regard to different RONS. Accordingly, the CAP parameters can be used to vary the RONS to be released in a reversible manner, without the need to remove or replace the nanomaterial.
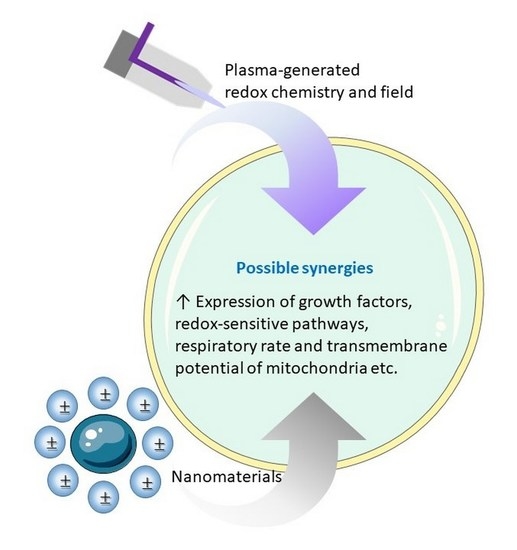
- Analogously, nanomaterials may serve as RONS regulators or catalysts without being consumed, loaded or having their surface conditions altered. Inspiration on such mechanisms can be taken from the large body of knowledge on plasma catalysis [310,311,312]. However, similar to our remark on plasma surface modification in Section 4.4, plasma catalysis is mainly considered in the gas phase. Therefore, the available knowledge on heterogeneous (nano)catalysis in the liquid phase may be taken instead from the literature on advanced oxidation processes used in water treatment, in particular the plasma technology used for this purpose (see e.g., [313,314,315]). Another source of inspiration can be found in the available knowledge on the antioxidant and ROS-generating mechanisms for various NPs (Figure 10 and Figure 11).
- The plasma-induced redox chemistry can be used to modify the nanomaterial’s liquid environment, for instance in terms of pH and conductivity. In this way, the solubility, chemical activity or electrical behavior of the material becomes tunable in situ.
- Further, the combination of plasma and nanotechnology allows a dual enhancement in delivery mechanisms. On the one hand, NPs may serve as nanocarriers to transport plasma-generated RONS. On the other hand, CAP may modify the liquid medium to facilitate NP transport. A particular example is given by He et al., who found CAP to stimulate clathrin-dependent endocytosis, due to a repairment mechanism of oxidised cell membrane [144]. This enhanced the uptake of nanomaterial in glioblastoma multiforme cells.
- As mentioned on several occasions above, combining plasma and nanomedicine permits lowering the applied dose, while enhancing the treatment selectivity. With relation to RONS, each of the aforementioned points can be employed for this purpose.
4.6. Field-Related Synergies
5. Safety Concerns
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laroussi, M.; Bekeschus, S.; Keidar, M.; Bogaerts, A.; Fridman, A.; Lu, X.; Ostrikov, K.; Hori, M.; Stapelmann, K.; Miller, V. Low-Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 127–157. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Sahun, M.; Smits, E.; Bogaerts, A.; Privat-Maldonado, A. Modulating the Antioxidant Response for Better Oxidative Stress-Inducing Therapies: How to Take Advantage of Two Sides of the Same Medal? Biomedicines 2022, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Recek, N. Biocompatibility of plasma-treated polymeric implants. Materials 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Tabares, F.L.; Junkar, I. Cold Plasma Systems and Their Application in Surface Treatments for Medicine. Molecules 2021, 26, 1903. [Google Scholar] [CrossRef]
- Tan, F.; Fang, Y.; Zhu, L.; Al-Rubeai, M. Cold atmospheric plasma as an interface biotechnology for enhancing surgical implants. Crit. Rev. Biotechnol. 2021, 41, 425–440. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Linh, N.N.; Ghimire, B.; Pengkit, A.; Sornsakdanuphap, J.; Lee, S.-J.; Choi, E.H. Plasma and nanomaterials: Fabrication and biomedical applications. Nanomaterials 2019, 9, 98. [Google Scholar] [CrossRef]
- Dai, X.; Yu, L.; Zhao, X.; Ostrikov, K.K. Nanomaterials for oncotherapies targeting the hallmarks of cancer. Nanotechnology 2020, 31, 392001. [Google Scholar] [CrossRef]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Malyavko, A.; Wang, Q.; Lin, L.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma cancer treatment, a critical review. Appl. Sci. 2021, 11, 7757. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, F.; Vanraes, P.; Nikiforov, A.; Morent, R.; De Geyter, N. Applications of plasma-liquid systems: A review. Materials 2019, 12, 2751. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma medicine: Applications of cold atmospheric pressure plasma in dermatology. Oxidative Med. Cell. Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef]
- Gan, L.; Jiang, J.; Duan, J.W.; Wu, X.J.Z.; Zhang, S.; Duan, X.R.; Song, J.Q.; Chen, H.X. Cold atmospheric plasma applications in dermatology: A systematic review. J. Biophotonics 2021, 14, e202000415. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Bekeschus, S. Redox for Repair: Cold Physical Plasmas and Nrf2 Signaling Promoting Wound Healing. Antioxidants 2018, 7, 146. [Google Scholar] [CrossRef]
- Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B.; et al. Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment. Appl. Sci. 2020, 10, 6898. [Google Scholar] [CrossRef]
- Li, H.P.; Zhang, X.F.; Zhu, X.M.; Zheng, M.; Liu, S.F.; Qi, X.; Wang, K.P.; Chen, J.; Xi, X.Q.; Tan, J.G. Translational plasma stomatology: Applications of cold atmospheric plasmas in dentistry and their extension. High Volt. 2017, 2, 188–199. [Google Scholar] [CrossRef]
- Borges, A.C.; Kostov, K.G.; Pessoa, R.S.; de Abreu, G.; Lima, G.d.M.; Figueira, L.W.; Koga-Ito, C.Y. Applications of cold atmospheric pressure plasma in dentistry. Appl. Sci. 2021, 11, 1975. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Kwak, H.S.; Park, J.H.; Uhm, H.S.; Bogaerts, A.; Choi, E.H.; Attri, P. Bacterial inactivation by plasma treated water enhanced by reactive nitrogen species. Sci. Rep. 2018, 8, 11268. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Hammerschmid, D.; Privat-Maldonado, A.; Dewilde, S.; Bogaerts, A. Synergistic effects of melittin and plasma treatment: A promising approach for cancer therapy. Cancers 2019, 11, 1109. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Joshi, K.; Mazumder, B.; Chattopadhyay, P.; Bora, N.S.; Goyary, D.; Karmakar, S. Graphene family of nanomaterials: Reviewing advanced applications in drug delivery and medicine. Curr. Drug Deliv. 2019, 16, 195–214. [Google Scholar] [CrossRef]
- Tang, L.; He, S.; Yin, Y.; Liu, H.; Hu, J.; Cheng, J.; Wang, W. Combination of Nanomaterials in Cell-Based Drug Delivery Systems for Cancer Treatment. Pharmaceutics 2021, 13, 1888. [Google Scholar] [CrossRef]
- Qi, G.B.; Gao, Y.J.; Wang, L.; Wang, H. Self-assembled peptide-based nanomaterials for biomedical imaging and therapy. Adv. Mater. 2018, 30, 1703444. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Durairaj, S.; Sidhureddy, B.; Cirone, J.; Chen, A. Nanomaterials-based electrochemical sensors for in vitro and in vivo analyses of neurotransmitters. Appl. Sci. 2018, 8, 1504. [Google Scholar] [CrossRef]
- Song, G.; Zhao, M.; Chen, H.; Lenahan, C.; Zhou, X.; Ou, Y.; He, Y. The role of nanomaterials in stroke treatment: Targeting oxidative stress. Oxidative Med. Cell. Longev. 2021, 2021, 8857486. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Sun, X. Reactive oxygen species-based nanomaterials for cancer therapy. Front. Chem. 2021, 9, 650587. [Google Scholar] [CrossRef]
- Liu, P.; Huo, M.; Shi, J. Nanocatalytic Medicine of Iron-Based Nanocatalysts. CCS Chem. 2021, 3, 2445–2463. [Google Scholar] [CrossRef]
- Cormode, D.P.; Gao, L.; Koo, H. Emerging biomedical applications of enzyme-like catalytic nanomaterials. Trends Biotechnol. 2018, 36, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yang, F.; Nie, C.; Ma, L.; Cheng, C.; Haag, R. Biocatalytic nanomaterials: A new pathway for bacterial disinfection. Adv. Mater. 2021, 33, 2100637. [Google Scholar] [CrossRef] [PubMed]
- Ferson, N.D.; Uhl, A.M.; Andrew, J.S. Piezoelectric and magnetoelectric scaffolds for tissue regeneration and biomedicine: A review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 68, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Chen, S.; Zhang, H.; Zhou, J.; Fan, H.M.; Liang, X.J. Magnetic nanomaterials for advanced regenerative medicine: The promise and challenges. Adv. Mater. 2019, 31, 1804922. [Google Scholar] [CrossRef]
- Kong, M.; Keidar, M.; Ostrikov, K. Plasmas meet nanoparticles—Where synergies can advance the frontier of medicine. J. Phys. D Appl. Phys. 2011, 44, 174018. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015, 24, 033001. [Google Scholar] [CrossRef]
- Keidar, M. Therapeutic approaches based on plasmas and nanoparticles. J. Nanomed. Res. 2016, 3, 00052. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977. [Google Scholar] [CrossRef]
- Aryal, S.; Bisht, G. New paradigm for a targeted cancer therapeutic approach: A short review on potential synergy of gold nanoparticles and cold atmospheric plasma. Biomedicines 2017, 5, 38. [Google Scholar] [CrossRef]
- Smolková, B.; Uzhytchak, M.; Lynnyk, A.; Kubinová, Š.; Dejneka, A.; Lunov, O. A critical review on selected external physical cues and modulation of cell behavior: Magnetic nanoparticles, non-thermal plasma and lasers. J. Funct. Biomater. 2018, 10, 2. [Google Scholar] [CrossRef]
- Liu, D.; Szili, E.J.; Ostrikov, K. Plasma medicine: Opportunities for nanotechnology in a digital age. Plasma Process. Polym. 2020, 17, 2000097. [Google Scholar] [CrossRef]
- Rasouli, M.; Fallah, N.; Bekeschus, S. Combining Nanotechnology and Gas Plasma as an Emerging Platform for Cancer Therapy: Mechanism and Therapeutic Implication. Oxidative Med. Cell. Longev. 2021, 2021, 2990326. [Google Scholar] [CrossRef]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef]
- Hill, M.J.; Qi, B.; Bayaniahangar, R.; Araban, V.; Bakhtiary, Z.; Doschak, M.R.; Goh, B.C.; Shokouhimehr, M.; Vali, H.; Presley, J.F.; et al. Nanomaterials for bone tissue regeneration: Updates and future perspectives. Nanomedicine 2019, 14, 2987–3006. [Google Scholar] [CrossRef]
- Kodama, J.; Harumningtyas, A.A.; Ito, T.; Michlíček, M.; Sugimoto, S.; Kita, H.; Chijimatsu, R.; Ukon, Y.; Kushioka, J.; Okada, R.; et al. Amine modification of calcium phosphate by low-pressure plasma for bone regeneration. Sci. Rep. 2021, 11, 17870. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, A.; Toyoda, H.; Orita, K.; Hirakawa, Y.; Aoki, K.; Oh, J.-S.; Shirafuji, T.; Nakamura, H. In vivo study on the healing of bone defect treated with non-thermal atmospheric pressure gas discharge plasma. PLoS ONE 2021, 16, e0255861. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Aadil, K.R.; Ranjan, S.; Kumar, V.B. Advances in nanotechnology and nanomaterials based strategies for neural tissue engineering. J. Drug Deliv. Sci. Technol. 2020, 57, 101617. [Google Scholar] [CrossRef]
- Lee, H.-G.; Choi, J.-H.; Jang, Y.-S.; Kim, U.-K.; Kim, G.-C.; Hwang, D.-S. Non-thermal plasma accelerates the healing process of peripheral nerve crush injury in rats. Int. J. Med. Sci. 2020, 17, 1112. [Google Scholar] [CrossRef]
- Lee, S.-T.; Jang, Y.-S.; Kim, U.-K.; Kim, H.-J.; Ryu, M.-H.; Kim, G.-C.; Hwang, D.-S. Non-thermal plasma application enhances the recovery of transected sciatic nerves in rats. Exp. Biol. Med. 2021, 246, 1287–1296. [Google Scholar] [CrossRef]
- Mitra, S.; Kaushik, N.; Moon, I.S.; Choi, E.H.; Kaushik, N.K. Utility of Reactive Species Generation in Plasma Medicine for Neuronal Development. Biomedicines 2020, 8, 348. [Google Scholar] [CrossRef]
- Borghi, F.F.; Rider, A.E.; Kumar, S.; Han, Z.J.; Haylock, D.; Ostrikov, K. Emerging stem cell controls: Nanomaterials and plasma effects. J. Nanomater. 2013, 2013, 329139. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Moghaddam, A.; Manouchehri, S.; Atoufi, Z.; Amiri, A.; Amirkhani, M.; Nilforoushzadeh, M.; Saeb, M.; Hamblin, M.; Mozafari, M. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine 2017, 12, 2403–2422. [Google Scholar] [CrossRef] [PubMed]
- Emmert, S.; Pantermehl, S.; Foth, A.; Waletzko-Hellwig, J.; Hellwig, G.; Bader, R.; Illner, S.; Grabow, N.; Bekeschus, S.; Weltmann, K.-D.; et al. Combining Biocompatible and Biodegradable Scaffolds and Cold Atmospheric Plasma for Chronic Wound Regeneration. Int. J. Mol. Sci. 2021, 22, 9199. [Google Scholar] [CrossRef] [PubMed]
- Gilgenkrantz, H.; de l’Hortet, A.C. Understanding liver regeneration: From mechanisms to regenerative medicine. Am. J. Pathol. 2018, 188, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles used in dentistry: A review. J. Oral Biol. Craniofac. Res. 2018, 8, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Wang, M. Fabrication of HA/PHBV composite scaffolds through the emulsion freezing/freeze-drying process and characterisation of the scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Khan, T.H. In vitro degradation of PHBV scaffolds and nHA/PHBV composite scaffolds containing hydroxyapatite nanoparticles for bone tissue engineering. J. Nanomater. 2012, 2012, 1. [Google Scholar]
- McKeon-Fischer, K.; Freeman, J. Characterization of electrospun poly (L-lactide) and gold nanoparticle composite scaffolds for skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 560–568. [Google Scholar] [CrossRef]
- Chen, J.; Yu, M.; Guo, B.; Ma, P.X.; Yin, Z. Conductive nanofibrous composite scaffolds based on in-situ formed polyaniline nanoparticle and polylactide for bone regeneration. J. Colloid Interface Sci. 2018, 514, 517–527. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Tee, J.K.; Ong, C.N.; Bay, B.H.; Ho, H.K.; Leong, D.T. Oxidative stress by inorganic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 414–438. [Google Scholar] [CrossRef]
- Mauricio, M.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.; Rocha, M.; Vila, J.; Victor, V. Nanoparticles in medicine: A focus on vascular oxidative stress. Oxidative Med. Cell. Longev. 2018, 2018, 6231482. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Pathak, S.; Castagliuolo, I.; Palù, G.; Brun, P.; Zuin, M.; Cavazzana, R.; Martines, E. Helium generated cold plasma finely regulates activation of human fibroblast-like primary cells. PLoS ONE 2014, 9, e104397. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Attri, P.; Dewilde, S.; Bogaerts, A. Inactivation of human pancreatic ductal adenocarcinoma with atmospheric plasma treated media and water: A comparative study. J. Phys. D Appl. Phys. 2018, 51, 255401. [Google Scholar] [CrossRef]
- Kaushik, N.; Kumar, N.; Kim, C.H.; Kaushik, N.K.; Choi, E.H. Dielectric barrier discharge plasma efficiently delivers an apoptotic response in human monocytic lymphoma. Plasma Process. Polym. 2014, 11, 1175–1187. [Google Scholar] [CrossRef]
- Kumar, N.; Perez-Novo, C.; Shaw, P.; Logie, E.; Privat-Maldonado, A.; Dewilde, S.; Smits, E.; Berghe, W.V.; Bogaerts, A. Physical plasma-derived oxidants sensitize pancreatic cancer cells to ferroptotic cell death. Free Radic. Biol. Med. 2021, 166, 187–200. [Google Scholar] [CrossRef]
- Kang, E.-S.; Kim, D.-S.; Han, Y.; Son, H.; Chung, Y.-H.; Min, J.; Kim, T.-H. Three-dimensional graphene–RGD peptide nanoisland composites that enhance the osteogenesis of human adipose-derived mesenchymal stem cells. Int. J. Mol. Sci. 2018, 19, 669. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, D.; Zhang, J.; Fong, C.; Yang, M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater. Sci. Eng. C 2014, 42, 70–77. [Google Scholar] [CrossRef]
- Bapat, R.A.; Chaubal, T.V.; Dharmadhikari, S.; Abdulla, A.M.; Bapat, P.; Alexander, A.; Dubey, S.K.; Kesharwani, P. Recent advances of gold nanoparticles as biomaterial in dentistry. Int. J. Pharm. 2020, 586, 119596. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637. [Google Scholar] [CrossRef]
- Wei, M.; Li, S.; Le, W. Nanomaterials modulate stem cell differentiation: Biological interaction and underlying mechanisms. J. Nanobiotechnol. 2017, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Idumah, C.I. Progress in polymer nanocomposites for bone regeneration and engineering. Polym. Polym. Compos. 2021, 29, 509–527. [Google Scholar] [CrossRef]
- Vera-Sánchez, M.; Aznar-Cervantes, S.; Jover, E.; Garcia-Bernal, D.; Onate-Sánchez, R.E.; Hernández-Romero, D.; Moraleda, J.M.; Collado-González, M.; Rodríguez-Lozano, F.J.; Cenis, J.L. Silk-fibroin and graphene oxide composites promote human periodontal ligament stem cell spontaneous differentiation into osteo/cementoblast-like cells. Stem Cells Dev. 2016, 25, 1742–1754. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, D.Y.; Yang, Y.; Ha, H.; Lee, G.T.; Song, J.S.; Uh, S.-t.; Lee, H.B. Role of reactive oxygen species in TGF-β1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 2005, 16, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.-H.; Park, S.G.; Choi, J.-S.; Xia, Y.; Sung, J.-H. The pivotal role of reactive oxygen species generation in the hypoxia-induced stimulation of adipose-derived stem cells. Stem Cells Dev. 2011, 20, 1753–1761. [Google Scholar] [CrossRef]
- Trautwein, C.; Rakemann, T.; Niehof, M.; Rose-John, S.; Manns, M.P. Acute-phase response factor, increased binding, and target gene transcription during liver regeneration. Gastroenterology 1996, 110, 1854–1862. [Google Scholar] [CrossRef]
- Serras, F. The benefits of oxidative stress for tissue repair and regeneration. Fly 2016, 10, 128–133. [Google Scholar] [CrossRef]
- Tong, Y.F.; Liu, Y.; Hu, Z.X.; Li, Z.C. Protocatechuic aldehyde inhibits TNF-α-induced fibronectin expression in human umbilical vein endothelial cells via a c-Jun N-terminal kinase dependent pathway. Exp. Ther. Med. 2016, 11, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Kita, M.; Yamamoto, K.; Akamatsu, Y.; Oseko, F.; Kanamura, N. Mechanical stress enhances production of cytokines in human periodontal ligament cells induced by Porphyromonas gingivalis. Arch. Oral Biol. 2011, 56, 251–257. [Google Scholar] [CrossRef]
- Kim, I.S.; Song, J.K.; Song, Y.M.; Cho, T.H.; Lee, T.H.; Lim, S.S.; Kim, S.J.; Hwang, S.J. Novel effect of biphasic electric current on in vitro osteogenesis and cytokine production in human mesenchymal stromal cells. Tissue Eng. Part A 2009, 15, 2411–2422. [Google Scholar] [CrossRef]
- Simon, A.R.; Rai, U.; Fanburg, B.L.; Cochran, B.H. Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol.-Cell Physiol. 1998, 275, C1640–C1652. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Kamimura, M.; Bea, F.; Akizawa, T.; Katus, H.A.; Kreuzer, J.; Viedt, C. Platelet-derived growth factor induces tissue factor expression in vascular smooth muscle cells via activation of Egr-1. Hypertension 2004, 44, 944–951. [Google Scholar] [CrossRef]
- Ji, A.-R.; Ku, S.-Y.; Cho, M.S.; Kim, Y.Y.; Kim, Y.J.; Oh, S.K.; Kim, S.H.; Moon, S.Y.; Choi, Y.M. Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp. Mol. Med. 2010, 42, 175–186. [Google Scholar] [CrossRef]
- Morrison, M. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. [Google Scholar] [CrossRef]
- Lee, R.C.; Canaday, D.J.; Doong, H. A review of the biophysical basis for the clinical application of electric fields in soft-tissue repair. J. Burn Care Rehabil. 1993, 14, 319–335. [Google Scholar] [CrossRef]
- Lozano, D.; Gonzales-Portillo, G.S.; Acosta, S.; de la Pena, I.; Tajiri, N.; Kaneko, Y.; Borlongan, C.V. Neuroinflammatory responses to traumatic brain injury: Etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 2015, 11, 97. [Google Scholar]
- Jo, E.; Seo, G.; Kwon, J.-T.; Lee, M.; cheun Lee, B.; Eom, I.; Kim, P.; Choi, K. Exposure to zinc oxide nanoparticles affects reproductive development and biodistribution in offspring rats. J. Toxicol. Sci. 2013, 38, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Yusupov, M.; Privat-Maldonado, A.; Cordeiro, R.M.; Verswyvel, H.; Shaw, P.; Razzokov, J.; Smits, E.; Bogaerts, A. Oxidative damage to hyaluronan–CD44 interactions as an underlying mechanism of action of oxidative stress-inducing cancer therapy. Redox Biol. 2021, 43, 101968. [Google Scholar] [CrossRef]
- Hoon Park, J.; Kumar, N.; Hoon Park, D.; Yusupov, M.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A.; Ho Kang, M.; Sup Uhm, H.; Ha Choi, E.; et al. A comparative study for the inactivation of multidrug resistance bacteria using dielectric barrier discharge and nano-second pulsed plasma. Sci. Rep. 2015, 5, 13849. [Google Scholar] [CrossRef]
- Stoffels, E.; Flikweert, A.; Stoffels, W.; Kroesen, G. Plasma needle: A non-destructive atmospheric plasma source for fine surface treatment of (bio) materials. Plasma Sources Sci. Technol. 2002, 11, 383. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.-D. Plasma medicine: A field of applied redox biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef]
- Girard, P.-M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic effect of H2O2 and NO2 in cell death induced by cold atmospheric He plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Privat-Maldonado, A.; Smits, E.; Bogaerts, A. Cold atmospheric plasma increases temozolomide sensitivity of three-dimensional glioblastoma spheroids via oxidative stress-mediated DNA damage. Cancers 2021, 13, 1780. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, K.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Kajiyama, H.; Utsumi, F.; Kikkawa, F.; Hori, M. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 2016, 6, 36282. [Google Scholar] [CrossRef]
- Zubor, P.; Wang, Y.; Liskova, A.; Samec, M.; Koklesova, L.; Dankova, Z.; Dørum, A.; Kajo, K.; Dvorska, D.; Lucansky, V.; et al. Cold Atmospheric Pressure Plasma (CAP) as a New Tool for the Management of Vulva Cancer and Vulvar Premalignant Lesions in Gynaecological Oncology. Int. J. Mol. Sci. 2020, 21, 7988. [Google Scholar] [CrossRef]
- Kim, G.; Kim, G.; Park, S.; Jeon, S.; Seo, H.; Iza, F.; Lee, J.K. Air plasma coupled with antibody-conjugated nanoparticles: A new weapon against cancer. J. Phys. D Appl. Phys. 2008, 42, 032005. [Google Scholar] [CrossRef]
- Kumar, N.; Attri, P.; Yadav, D.K.; Choi, J.; Choi, E.H.; Uhm, H.S. Induced apoptosis in melanocytes cancer cell and oxidation in biomolecules through deuterium oxide generated from atmospheric pressure non-thermal plasma jet. Sci. Rep. 2014, 4, 7589. [Google Scholar] [CrossRef]
- Cheng, X.; Murphy, W.; Recek, N.; Yan, D.; Cvelbar, U.; Vesel, A.; Mozetič, M.; Canady, J.; Keidar, M.; Sherman, J.H. Synergistic effect of gold nanoparticles and cold plasma on glioblastoma cancer therapy. J. Phys. D Appl. Phys. 2014, 47, 335402. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Sherman, J.H.; Cheng, X.; Keidar, M. Toward understanding the selective anticancer capacity of cold atmospheric plasma—A model based on aquaporins. Biointerphases 2015, 10, 040801. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, X.; Lin, L.; Keidar, M. Cold atmospheric plasma discharged in water and its potential use in cancer therapy. J. Phys. D Appl. Phys. 2016, 50, 015208. [Google Scholar] [CrossRef]
- Bruggeman, P.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.; Graham, W.; Graves, D.B.; Hofman-Caris, R.; Maric, D.; Reid, J.P.; Ceriani, E. Plasma–liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Van Boxem, W.; Van der Paal, J.; Gorbanev, Y.; Vanuytsel, S.; Smits, E.; Dewilde, S.; Bogaerts, A. Anti-cancer capacity of plasma-treated PBS: Effect of chemical composition on cancer cell cytotoxicity. Sci. Rep. 2017, 7, 16478. [Google Scholar] [CrossRef]
- Choi, B.B.R.; Choi, J.H.; Hong, J.W.; Song, K.W.; Lee, H.J.; Kim, U.K.; Kim, G.C. Selective killing of melanoma cells with non-thermal atmospheric pressure plasma and p-FAK antibody conjugated gold nanoparticles. Int. J. Med. Sci. 2017, 14, 1101. [Google Scholar] [CrossRef]
- Furuta, T.; Shi, L.; Toyokuni, S. Non-thermal plasma as a simple ferroptosis inducer in cancer cells: A 4 possible role of ferritin 5. Pathol. Int. (Lett. Ed.) 2018, 2, 3. [Google Scholar] [CrossRef]
- Yusupov, M.; Yan, D.; Cordeiro, R.M.; Bogaerts, A. Atomic scale simulation of H2O2permeation through aquaporin: Toward the understanding of plasma cancer treatment. J. Phys. D Appl. Phys. 2018, 51, 125401. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Wang, S.; Li, X.; Li, W.; Ding, D.; Gong, X.; Keidar, M.; Zhang, W. Paclitaxel-loaded core–shell magnetic nanoparticles and cold atmospheric plasma inhibit non-small cell lung cancer growth. ACS Appl. Mater. Interfaces 2018, 10, 43462–43471. [Google Scholar] [CrossRef]
- Bogaerts, A.; Yusupov, M.; Razzokov, J.; Van der Paal, J. Plasma for cancer treatment: How can rons penetrate through the cell membrane? Answers from computer modeling. Front. Chem. Sci. Eng. 2019, 13, 253–263. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.U.; Morfill, G.E.; Klämpfl, T.G.; Steffes, B.; Thomas, H.M.; et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Chuangsuwanich, A.; Assadamongkol, T.; Boonyawan, D. The Healing Effect of Low-Temperature Atmospheric-Pressure Plasma in Pressure Ulcer: A Randomized Controlled Trial. Int. J. Low. Extrem. Wounds 2016, 15, 313–319. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.-C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Gangal, U.; Youssef, M.M.; Goyal, S.M.; Bruggeman, P.J. Inactivation of virus in solution by cold atmospheric pressure plasma: Identification of chemical inactivation pathways. J. Phys. D Appl. Phys. 2016, 49, 204001. [Google Scholar] [CrossRef]
- Bunz, O.; Mese, K.; Funk, C.; Wulf, M.; Bailer, S.M.; Piwowarczyk, A.; Ehrhardt, A. Cold atmospheric plasma as antiviral therapy–effect on human herpes simplex virus type 1. J. Gen. Virol. 2020, 101, 208. [Google Scholar] [CrossRef]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef]
- Jablonowski, H.; Hänsch, M.A.C.; Dünnbier, M.; Wende, K.; Hammer, M.U.; Weltmann, K.-D.; Reuter, S.; von Woedtke, T. Plasma jet’s shielding gas impact on bacterial inactivation. Biointerphases 2015, 10, 029506. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Min, B.; Choi, K.H.; Hong, Y.J.; Miller, V.; Fridman, A.; Choi, E.H. Cytotoxic macrophage-released tumour necrosis factor-alpha (TNF-α) as a killing mechanism for cancer cell death after cold plasma activation. J. Phys. D Appl. Phys. 2016, 49, 084001. [Google Scholar] [CrossRef]
- Aguirre, J.; Ríos-Momberg, M.; Hewitt, D.; Hansberg, W. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 2005, 13, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Suzuki, Y.J. Cell proliferation, reactive oxygen and cellular glutathione. Dose-Response 2005, 3. [Google Scholar] [CrossRef]
- Veal, E.; Day, A. Hydrogen peroxide as a signaling molecule. Antioxid. Redox Signal. 2011, 15, 147–151. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Suh, Y.-A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82. [Google Scholar] [CrossRef]
- Weinberg, F.; Chandel, N.S. Reactive oxygen species-dependent signaling regulates cancer. Cell. Mol. Life Sci. 2009, 66, 3663. [Google Scholar] [CrossRef]
- Knebel, A.; Rahmsdorf, H.J.; Ullrich, A.; Herrlich, P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 1996, 15, 5314–5325. [Google Scholar] [CrossRef]
- Esposito, F.; Chirico, G.; Gesualdi, N.M.; Posadas, I.; Ammendola, R.; Russo, T.; Cirino, G.; Cimino, F. Protein kinase B activation by reactive oxygen species is independent of tyrosine kinase receptor phosphorylation and requires SRC activity. J. Biol. Chem. 2003, 278, 20828–20834. [Google Scholar] [CrossRef]
- Klimova, T.; Chandel, N. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008, 15, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Staleva, L.; Hall, A.; Orlow, S.J. Oxidative stress activates FUS1 and RLM1 transcription in the yeast Saccharomyces cerevisiae in an oxidant-dependent manner. Mol. Biol. Cell 2004, 15, 5574–5582. [Google Scholar] [CrossRef]
- Bilsland, E.; Molin, C.; Swaminathan, S.; Ramne, A.; Sunnerhagen, P. Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol. Microbiol. 2004, 53, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.-C.; Buckley, D.A.; Galic, S.; Tiganis, T.; Tonks, N.K. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J. Biol. Chem. 2004, 279, 37716–37725. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikawa, S.; Yoshizawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J. Phys. D Appl. Phys. 2014, 47, 285403. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Alexander, R.W.; Akers, M.; Yin, Q.; Fujio, Y.; Walsh, K.; Griendling, K.K. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 1999, 274, 22699–22704. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Suh, D.; Tang, T.; Lee, H.J.; Roe, J.-S.; Kim, G.C.; Han, S.; Song, K. Non-thermal atmospheric pressure plasma induces epigenetic modifications that activate the expression of various cytokines and growth factors in human mesoderm-derived stem cells. Free Radic. Biol. Med. 2020, 148, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Shaw, P.; Razzokov, J.; Yusupov, M.; Attri, P.; Uhm, H.S.; Choi, E.H.; Bogaerts, A. Enhancement of cellular glucose uptake by reactive species: A promising approach for diabetes therapy. RSC Adv. 2018, 8, 9887–9894. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, J.; Liu, D.; Liu, S.; Lei, D.; Zheng, L.; Wei, Q.; Gao, M. Zinc-based metal organic framework with antibacterial and anti-inflammatory properties for promoting wound healing. Regen. Biomater. 2022, 9, rbac019. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Tornín, J.; Ginebra, M.-P.; Canal, C. Cold Atmospheric Plasma: A New Strategy Based Primarily on Oxidative Stress for Osteosarcoma Therapy. J. Clin. Med. 2021, 10, 893. [Google Scholar] [CrossRef]
- Yan, X.; Ouyang, J.; Zhang, C.; Shi, Z.; Wang, B.; Ostrikov, K. Plasma medicine for neuroscience—An introduction. Chin. Neurosurg. J. 2019, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-Y.; Hong, Y.J.; Lim, J.; Choi, J.S.; Choi, E.H.; Kang, S.; Rhim, H. Cold atmospheric plasma (CAP), a novel physicochemical source, induces neural differentiation through cross-talk between the specific RONS cascade and Trk/Ras/ERK signaling pathway. Biomaterials 2018, 156, 258–273. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Hu, Y.; Nie, T.; Tang, H.; Zhu, J.; Chen, K.; Liu, L.; Leong, K.W.; Chen, Y.; Mao, H.-Q. Size-controlled lipid nanoparticle production using turbulent mixing to enhance oral DNA delivery. Acta Biomater. 2018, 81, 195–207. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, K.; Scally, L.; Manaloto, E.; Gunes, S.; Ng, S.W.; Maher, M.; Tiwari, B.; Byrne, H.J.; Bourke, P. Cold atmospheric plasma stimulates clathrin-dependent endocytosis to repair oxidised membrane and enhance uptake of nanomaterial in glioblastoma multiforme cells. Sci. Rep. 2020, 10, 6985. [Google Scholar] [CrossRef] [PubMed]
- Haralambiev, L.; Neuffer, O.; Nitsch, A.; Kross, N.C.; Bekeschus, S.; Hinz, P.; Mustea, A.; Ekkernkamp, A.; Gümbel, D.; Stope, M.B. Inhibition of angiogenesis by treatment with cold atmospheric plasma as a promising therapeutic approach in oncology. Int. J. Mol. Sci. 2020, 21, 7098. [Google Scholar] [CrossRef] [PubMed]
- Motaln, H.; Recek, N.; Rogelj, B. Intracellular responses triggered by cold atmospheric plasma and plasma-activated media in cancer cells. Molecules 2021, 26, 1336. [Google Scholar] [CrossRef]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Barcia, C.; Conway, G.E.; Cullen, P.J. Cold atmospheric plasma induces ATP-dependent endocytosis of nanoparticles and synergistic U373MG cancer cell death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef]
- Kim, H.R.; Andrieux, K.; Gil, S.; Taverna, M.; Chacun, H.; Desmaële, D.; Taran, F.; Georgin, D.; Couvreur, P. Translocation of poly (ethylene glycol-co-hexadecyl) cyanoacrylate nanoparticles into rat brain endothelial cells: Role of apolipoproteins in receptor-mediated endocytosis. Biomacromolecules 2007, 8, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C. Electroporation of cells and tissues. IEEE Trans. Plasma Sci. 2000, 28, 24–33. [Google Scholar] [CrossRef]
- Nuccitelli, R. Application of pulsed electric fields to cancer therapy. Bioelectricity 2019, 1, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Vanraes, P.; Bogaerts, A. The essential role of the plasma sheath in plasma–liquid interaction and its applications—A perspective. J. Appl. Phys. 2021, 129, 220901. [Google Scholar] [CrossRef]
- Vanraes, P.; Bogaerts, A. Plasma physics of liquids—A focused review. Appl. Phys. Rev. 2018, 5, 031103. [Google Scholar] [CrossRef]
- Elg, D.T.; Delgado, H.E.; Martin, D.C.; Sankaran, R.M.; Rumbach, P.; Bartels, D.M.; Go, D.B. Recent advances in understanding the role of solvated electrons at the plasma-liquid interface of solution-based gas discharges. Spectrochim. Acta Part B At. Spectrosc. 2021, 186, 106307. [Google Scholar] [CrossRef]
- Boeuf, J.; Yang, L.; Pitchford, L. Dynamics of a guided streamer (‘plasma bullet’) in a helium jet in air at atmospheric pressure. J. Phys. D Appl. Phys. 2012, 46, 015201. [Google Scholar] [CrossRef]
- Sretenović, G.B.; Krstić, I.B.; Kovačević, V.V.; Obradović, B.M.; Kuraica, M.M. Spatio-temporally resolved electric field measurements in helium plasma jet. J. Phys. D Appl. Phys. 2014, 47, 102001. [Google Scholar] [CrossRef]
- Lu, X.; Laroussi, M. Dynamics of an atmospheric pressure plasma plume generated by submicrosecond voltage pulses. J. Appl. Phys. 2006, 100, 063302. [Google Scholar] [CrossRef]
- Pei, X.; Lu, Y.; Wu, S.; Xiong, Q.; Lu, X. A study on the temporally and spatially resolved OH radical distribution of a room-temperature atmospheric-pressure plasma jet by laser-induced fluorescence imaging. Plasma Sources Sci. Technol. 2013, 22, 025023. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Liao, Z.; Pei, X.; Wu, S. OH radicals distribution and discharge dynamics of an atmospheric pressure plasma jet above water surface. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 2, 223–228. [Google Scholar] [CrossRef]
- Kogelschatz, U. Filamentary, patterned, and diffuse barrier discharges. IEEE Trans. Plasma Sci. 2002, 30, 1400–1408. [Google Scholar] [CrossRef]
- Wagner, H.-E.; Brandenburg, R.; Kozlov, K.; Sonnenfeld, A.; Michel, P.; Behnke, J. The barrier discharge: Basic properties and applications to surface treatment. Vacuum 2003, 71, 417–436. [Google Scholar]
- Vanraes, P.; Nikiforov, A.; Bogaerts, A.; Leys, C. Study of an AC dielectric barrier single micro-discharge filament over a water film. Sci. Rep. 2018, 8, 10919. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Golzio, M.; Rols, M.-P. Electric field-responsive nanoparticles and electric fields: Physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2019, 138, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Stacey, M.; Stickley, J.; Fox, P.; Statler, V.; Schoenbach, K.; Beebe, S.; Buescher, S. Differential effects in cells exposed to ultra-short, high intensity electric fields: Cell survival, DNA damage, and cell cycle analysis. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2003, 542, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Garner, A.L.; Chen, G.; Jing, Y.; Deng, Y.; Swanson, R.J.; Kolb, J.F.; Beebe, S.J.; Joshi, R.P.; Schoenbach, K.H. Nanosecond electric pulses penetrate the nucleus and enhance speckle formation. Biochem. Biophys. Res. Commun. 2007, 364, 220–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Xiong, Z.-A.; Chen, W.-J.; Yao, C.G.; Zhao, Z.-Y.; Hua, Y.-Y. Intense picosecond pulsed electric fields inhibit proliferation and induce apoptosis of HeLa cells. Mol. Med. Rep. 2013, 7, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.-Y.; Wang, X.-S.; Zhang, Y.; Yao, C.-G.; Zhang, X.-M.; Xiong, Z.-A. Intense picosecond pulsed electric fields induce apoptosis through a mitochondrial-mediated pathway in HeLa cells. Mol. Med. Rep. 2012, 5, 981–987. [Google Scholar] [CrossRef]
- Mao, Z.; Liu, L.; Zhang, Y.; Zhang, J.; Liu, N.; Liu, Q.H. Selective electroporation of organelles under an intense picosecond pulsed electric field. IEEE J. Multiscale Multiphys. Comput. Tech. 2018, 3, 235–245. [Google Scholar] [CrossRef]
- Jia, J.; Xiong, Z.A.; Qin, Q.; Yao, C.G.; Zhao, X.Z. Picosecond pulsed electric fields induce apoptosis in a cervical cancer xenograft. Mol. Med. Rep. 2015, 11, 1623–1628. [Google Scholar] [CrossRef]
- Matveyenko, O.A.; Komnatnov, M.E.; Busygina, A.V.; Zharkova, L.P. Study of impact of picosecond pulses on functional status of mitochondria of mice liver in TEM-cell. In Proceedings of the 2016 17th International Conference of Young Specialists on Micro/Nanotechnologies and Electron Devices (EDM), Erlagol, Russia, 30 June–4 July 2016; pp. 657–660. [Google Scholar]
- Chen, W.-J.; Xiong, Z.-A.; Zhang, M.; Yao, C.-G.; Zhao, Z.-Y.; Hua, Y.-Y.; Zhou, W. Picosecond pulsed electric fields induce apoptosis in HeLa cells via the endoplasmic reticulum stress and caspase-dependent signaling pathways. Int. J. Oncol. 2013, 42, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Ouf, S.A.; Mohamed, A.A.H.; El-Sayed, W.S. Fungal decontamination of fleshy fruit water washes by double atmospheric pressure cold plasma. CLEAN–Soil Air Water 2016, 44, 134–142. [Google Scholar] [CrossRef]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.; Annapure, U. Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Tero, R.; Suda, Y.; Kato, R.; Tanoue, H.; Takikawa, H. Plasma irradiation of artificial cell membrane system at solid–liquid interface. Appl. Phys. Express 2014, 7, 077001. [Google Scholar] [CrossRef]
- Jinno, M.; Ikeda, Y.; Motomura, H.; Kido, Y.; Satoh, S. Investigation of plasma induced electrical and chemical factors and their contribution processes to plasma gene transfection. Arch. Biochem. Biophys. 2016, 605, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, M.; Van der Paal, J.; Neyts, E.C.; Bogaerts, A. Synergistic effect of electric field and lipid oxidation on the permeability of cell membranes. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Vijayarangan, V.; Delalande, A.; Dozias, S.; Pouvesle, J.-M.; Pichon, C.; Robert, E. Cold atmospheric plasma parameters investigation for efficient drug delivery in HeLa cells. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 2, 109–115. [Google Scholar] [CrossRef]
- Jinno, M.; Ikeda, Y.; Motomura, H.; Isozaki, Y.; Kido, Y.; Satoh, S. Synergistic effect of electrical and chemical factors on endocytosis in micro-discharge plasma gene transfection. Plasma Sources Sci. Technol. 2017, 26, 065016. [Google Scholar] [CrossRef]
- Haralambiev, L.; Nitsch, A.; Jacoby, J.M.; Strakeljahn, S.; Bekeschus, S.; Mustea, A.; Ekkernkamp, A.; Stope, M.B. Cold atmospheric plasma treatment of chondrosarcoma cells affects proliferation and cell membrane permeability. Int. J. Mol. Sci. 2020, 21, 2291. [Google Scholar] [CrossRef]
- Haralambiev, L.; Nitsch, A.; Einenkel, R.; Muzzio, D.O.; Gelbrich, N.; Burchardt, M.; Zygmunt, M.; Ekkernkamp, A.; Stope, M.B.; Guembel, D. The effect of cold atmospheric plasma on the membrane permeability of human osteosarcoma cells. Anticancer Res. 2020, 40, 841–846. [Google Scholar] [CrossRef]
- Foulds, I.; Barker, A. Human skin battery potentials and their possible role in wound healing. Br. J. Dermatol. 1983, 109, 515–522. [Google Scholar] [CrossRef]
- Tai, G.; Tai, M.; Zhao, M. Electrically stimulated cell migration and its contribution to wound healing. Burn. Trauma 2018, 6, 20. [Google Scholar] [CrossRef]
- Farber, P.L.; Isoldi, F.C.; Ferreira, L.M. Electric factors in wound healing. Adv. Wound Care 2021, 10, 461–476. [Google Scholar] [CrossRef] [PubMed]
- McCaig, C.D.; Zhao, M. Physiological electrical fields modify cell behaviour. Bioessays 1997, 19, 819–826. [Google Scholar] [CrossRef]
- Iwasa, S.N.; Babona-Pilipos, R.; Morshead, C.M. Environmental factors that influence stem cell migration: An “electric field”. Stem Cells Int. 2017, 2017, 4276927. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, R.; Nuccitelli, P.; Li, C.; Narsing, S.; Pariser, D.M.; Lui, K. The electric field near human skin wounds declines with age and provides a noninvasive indicator of wound healing. Wound Repair Regen. 2011, 19, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, X.; Rajagopalan, P.; Zhang, L.; Zhan, S.; Huang, S.; Li, W.; Zeng, X.; Ye, Q.; Liu, Y. Toward controlled electrical stimulation for wound healing based on a precision layered skin model. ACS Appl. Bio Mater. 2020, 3, 8901–8910. [Google Scholar] [CrossRef]
- Hampton, S.; Collins, F. Treating a pressure ulcer with bio-electric stimulation therapy. Br. J. Nurs. 2006, 15, S14–S18. [Google Scholar] [CrossRef]
- Durand, D.M. Electric field effects in hyperexcitable neural tissue: A review. Radiat. Prot. Dosim. 2003, 106, 325–331. [Google Scholar] [CrossRef]
- Radman, T.; Su, Y.; An, J.H.; Parra, L.C.; Bikson, M. Spike timing amplifies the effect of electric fields on neurons: Implications for endogenous field effects. J. Neurosci. 2007, 27, 3030–3036. [Google Scholar] [CrossRef]
- Fröhlich, F.; McCormick, D.A. Endogenous electric fields may guide neocortical network activity. Neuron 2010, 67, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Shivacharan, R.S.; Chiang, C.-C.; Zhang, M.; Gonzalez-Reyes, L.E.; Durand, D.M. Self-propagating, non-synaptic epileptiform activity recruits neurons by endogenous electric fields. Exp. Neurol. 2019, 317, 119–128. [Google Scholar] [CrossRef]
- Modolo, J.; Denoyer, Y.; Wendling, F.; Benquet, P. Physiological effects of low-magnitude electric fields on brain activity: Advances from in vitro, in vivo and in silico models. Curr. Opin. Biomed. Eng. 2018, 8, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Anastassiou, C.A.; Perin, R.; Markram, H.; Koch, C. Ephaptic coupling of cortical neurons. Nat. Neurosci. 2011, 14, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Anastassiou, C.A.; Koch, C. Ephaptic coupling to endogenous electric field activity: Why bother? Curr. Opin. Neurobiol. 2015, 31, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Borgens, R.B. Embryonic neuroepithelial sodium transport, the resulting physiological potential, and cranial development. Dev. Biol. 1994, 165, 105–116. [Google Scholar] [CrossRef]
- Hotary, K.B.; Robinson, K.R. The neural tube of the Xenopus embryo maintains a potential difference across itself. Dev. Brain Res. 1991, 59, 65–73. [Google Scholar] [CrossRef]
- Hotary, K.B.; Robinson, K.R. Endogenous electrical currents and the resultant voltage gradients in the chick embryo. Dev. Biol. 1990, 140, 149–160. [Google Scholar] [CrossRef]
- Zhao, M.; Chalmers, L.; Cao, L.; Vieira, A.C.; Mannis, M.; Reid, B. Electrical signaling in control of ocular cell behaviors. Prog. Retin. Eye Res. 2012, 31, 65–88. [Google Scholar] [CrossRef]
- Jia, N.; Yang, J.; Liu, J.; Zhang, J. Electric Field: A Key Signal in Wound Healing. Chin. J. Plast. Reconstr. Surg. 2021, 3, 95–102. [Google Scholar] [CrossRef]
- Nuccitelli, R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat. Prot. Dosim. 2003, 106, 375–383. [Google Scholar] [CrossRef]
- Pullar, C.E.; Baier, B.S.; Kariya, Y.; Russell, A.J.; Horst, B.A.; Marinkovich, M.P.; Isseroff, R.R. β4 integrin and epidermal growth factor coordinately regulate electric field-mediated directional migration via Rac1. Mol. Biol. Cell 2006, 17, 4925–4935. [Google Scholar] [CrossRef]
- Hinkle, L.; McCaig, C.D.; Robinson, K.R. The direction of growth of differentiating neurones and myoblasts from frog embryos in an applied electric field. J. Physiol. 1981, 314, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Zhao, M.; Forrester, J.V.; McCaig, C.D. Re-orientation and Faster, Directed Migration of Lens Epithelial Cells in a Physiological Electric Field. Exp. Eye Res. 2000, 71, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Vanegas-Acosta, J.C.; Garzón-Alvarado, D.A.; Zwamborn, A.P.M. Mathematical model of electrotaxis in osteoblastic cells. Bioelectrochemistry 2012, 88, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Funk, R.H.W. Endogenous electric fields as guiding cue for cell migration. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funk, R.H. Does electromagnetic therapy meet an equivalent counterpart within the organism. J. Transl. Sci. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Li, L.; El-Hayek, Y.H.; Liu, B.; Chen, Y.; Gomez, E.; Wu, X.; Ning, K.; Li, L.; Chang, N.; Zhang, L.; et al. Direct-Current Electrical Field Guides Neuronal Stem/Progenitor Cell Migration. Stem Cells 2008, 26, 2193–2200. [Google Scholar] [CrossRef]
- Feng, J.F.; Liu, J.; Zhang, X.Z.; Zhang, L.; Jiang, J.Y.; Nolta, J.; Zhao, M. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells 2012, 30, 349–355. [Google Scholar] [CrossRef]
- Ariza, C.A.; Fleury, A.T.; Tormos, C.J.; Petruk, V.; Chawla, S.; Oh, J.; Sakaguchi, D.S.; Mallapragada, S.K. The Influence of Electric Fields on Hippocampal Neural Progenitor Cells. Stem Cell Rev. Rep. 2010, 6, 585–600. [Google Scholar] [CrossRef]
- Li, Y.; Weiss, M.; Yao, L. Directed migration of embryonic stem cell-derived neural cells in an applied electric field. Stem Cell Rev. Rep. 2014, 10, 653–662. [Google Scholar] [CrossRef]
- Hayashi, H.; Edin, F.; Li, H.; Liu, W.; Rask-Andersen, H. The effect of pulsed electric fields on the electrotactic migration of human neural progenitor cells through the involvement of intracellular calcium signaling. Brain Res. 2016, 1652, 195–203. [Google Scholar] [CrossRef]
- Arocena, M.; Zhao, M.; Collinson, J.M.; Song, B. A time-lapse and quantitative modelling analysis of neural stem cell motion in the absence of directional cues and in electric fields. J. Neurosci. Res. 2010, 88, 3267–3274. [Google Scholar] [CrossRef] [PubMed]
- McCaig, C.; Sangster, L.; Stewart, R. Neurotrophins enhance electric field-directed growth cone guidance and directed nerve branching. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2000, 217, 299–308. [Google Scholar] [CrossRef]
- Shan, Y.; Feng, H.; Li, Z. Electrical stimulation for nervous system injury: Research progress and prospects. Wuli Huaxue Xuebao/Acta Phys.-Chim. Sin. 2020, 36, 2005038-0. [Google Scholar] [CrossRef]
- Rajnicek, A.; Gow, N.; McCaig, C. Electric field-induced orientation of rat hippocampal neurones in vitro. Exp. Physiol. Transl. Integr. 1992, 77, 229–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; McCaig, C.D.; Agius-Fernandez, A.; Forrester, J.V.; Araki-Sasaki, K. Human corneal epithelial cells reorient and migrate cathodally in a small applied electric field. Curr. Eye Res. 1997, 16, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Agius-Fernandez, A.; Forrester, J.V.; McCaig, C.D. Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. J. Cell Sci. 1996, 109, 1405–1414. [Google Scholar] [CrossRef]
- Arai, K.Y.; Nakamura, Y.; Hachiya, Y.; Tsuchiya, H.; Akimoto, R.; Hosoki, K.; Kamiya, S.; Ichikawa, H.; Nishiyama, T. Pulsed electric current induces the differentiation of human keratinocytes. Mol. Cell. Biochem. 2013, 379, 235–241. [Google Scholar] [CrossRef]
- Jing, W.; Zhang, Y.; Cai, Q.; Chen, G.; Wang, L.; Yang, X.; Zhong, W. Study of electrical stimulation with different electric-field intensities in the regulation of the differentiation of PC12 cells. ACS Chem. Neurosci. 2018, 10, 348–357. [Google Scholar] [CrossRef]
- Dong, Z.-y.; Pei, Z.; Li, Z.; Wang, Y.-l.; Khan, A.; Meng, X.-t. Electric field stimulation induced neuronal differentiation of filum terminale derived neural progenitor cells. Neurosci. Lett. 2017, 651, 109–115. [Google Scholar] [CrossRef]
- Dong, Z.-y.; Pei, Z.; Wang, Y.-l.; Li, Z.; Khan, A.; Meng, X.-t. Ascl1 regulates electric field-induced neuronal differentiation through PI3K/Akt pathway. Neuroscience 2019, 404, 141–152. [Google Scholar] [CrossRef]
- Chang, H.-F.; Lee, Y.-S.; Tang, T.K.; Cheng, J.-Y. Pulsed DC electric field–induced differentiation of cortical neural precursor cells. PLoS ONE 2016, 11, e0158133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, Y.; Liu, J.; Yang, J.; Jia, N.; Zhu, C.; Zhang, J. Optimizing microenvironment by integrating negative pressure and exogenous electric fields via a flexible porous conductive dressing to accelerate wound healing. Biomater. Sci. 2021, 9, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics 2013, 34, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Li, S.K.; Jeong, E.-K.; Hastings, M.S. Magnetic resonance imaging study of current and ion delivery into the eye during transscleral and transcorneal iontophoresis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1224–1231. [Google Scholar] [CrossRef] [Green Version]
- Tomoda, K.; Terashima, H.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal delivery of indomethacin-loaded PLGA nanoparticles by iontophoresis. Colloids Surf. B Biointerfaces 2011, 88, 706–710. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kobayashi, S.; Hida, Y.; Makino, K. Estradiol-loaded PLGA nanoparticles for improving low bone mineral density of cancellous bone caused by osteoporosis: Application of enhanced charged nanoparticles with iontophoresis. Colloids Surf. B Biointerfaces 2017, 155, 35–40. [Google Scholar] [CrossRef]
- Moisescu, M.G.; Leveque, P.; Verjus, M.A.; Kovacs, E.; Mir, L.M. 900 MHz modulated electromagnetic fields accelerate the clathrin-mediated endocytosis pathway. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2009, 30, 222–230. [Google Scholar] [CrossRef]
- Mahrour, N.; Pologea-Moraru, R.; Moisescu, M.G.; Orlowski, S.; Levêque, P.; Mir, L.M. In vitro increase of the fluid-phase endocytosis induced by pulsed radiofrequency electromagnetic fields: Importance of the electric field component. Biochim. Biophys. Acta (BBA)-Biomembr. 2005, 1668, 126–137. [Google Scholar] [CrossRef]
- Chu, G.; Hayakawa, H.; Berg, P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987, 15, 1311–1326. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Lu, C. Electroporation of mammalian cells in a microfluidic channel with geometric variation. Anal. Chem. 2006, 78, 5158–5164. [Google Scholar] [CrossRef]
- Hammadi, Z.; Veesler, S. New approaches on crystallization under electric fields. Prog. Biophys. Mol. Biol. 2009, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M.; Avelar, Z.; Machado, L.; Pereira, R.N.; Vicente, A.A. Electric field effects on proteins–Novel perspectives on food and potential health implications. Food Res. Int. 2020, 137, 109709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, R.; Zhang, H.Q. Recent advances in the action of pulsed electric fields on enzymes and food component proteins. Trends Food Sci. Technol. 2012, 27, 83–96. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, Y.; Lu, L.; Chen, X.; Li, C. Pulsed electric fields processing of protein-based foods. Food Bioprocess Technol. 2014, 7, 114–125. [Google Scholar] [CrossRef]
- Zhao, M. Electrical fields in wound healing—An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J. Stem cell niches and endogenous electric fields in tissue repair. Front. Med. 2011, 5, 40–44. [Google Scholar] [CrossRef]
- Wang, E.-t.; Zhao, M. Regulation of tissue repair and regeneration by electric fields. Chin. J. Traumatol. 2010, 13, 55–61. [Google Scholar]
- Armstrong, P.F.; Brighton, C.T.; Star, A.M. Capacitively coupled electrical stimulation of bovine growth plate chondrocytes grown in pellet form. J. Orthop. Res. 1988, 6, 265–271. [Google Scholar] [CrossRef]
- Gratieri, T.; Kalaria, D.; Kalia, Y.N. Non-invasive iontophoretic delivery of peptides and proteins across the skin. Expert Opin. Drug Deliv. 2011, 8, 645–663. [Google Scholar] [CrossRef]
- Pikal, M.J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 2001, 46, 281–305. [Google Scholar] [CrossRef]
- Jadoul, A.; Bouwstra, J.; Preat, V. Effects of iontophoresis and electroporation on the stratum corneum: Review of the biophysical studies. Adv. Drug Deliv. Rev. 1999, 35, 89–105. [Google Scholar] [CrossRef]
- Viefhues, M.; Eichhorn, R. DNA dielectrophoresis: Theory and applications a review. Electrophoresis 2017, 38, 1483–1506. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, R.; Pethig, R. Protein dielectrophoresis: I. Status of experiments and an empirical theory. Micromachines 2020, 11, 533. [Google Scholar] [CrossRef]
- Kim, D.; Sonker, M.; Ros, A. Dielectrophoresis: From molecular to micrometer-scale analytes. Anal. Chem. 2018, 91, 277–295. [Google Scholar] [CrossRef]
- Zhao, S.; Mehta, A.S.; Zhao, M. Biomedical applications of electrical stimulation. Cell. Mol. Life Sci. 2020, 77, 2681–2699. [Google Scholar] [CrossRef] [PubMed]
- Eccles, N.K. A critical review of randomized controlled trials of static magnets for pain relief. J. Altern. Complement. Med. 2005, 11, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shang, P. A review of bioeffects of static magnetic field on rodent models. Prog. Biophys. Mol. Biol. 2014, 114, 14–24. [Google Scholar] [CrossRef]
- Markov, M.S. Therapeutic application of static magnetic fields. Environ. 2007, 27, 457–463. [Google Scholar] [CrossRef]
- Zhang, X.; Yarema, K.; Xu, A. Static Magnetic Fields (SMFs) on Human Bodies. In Biological Effects of Static Magnetic Fields; Springer: Singapore, 2017; pp. 27–47. [Google Scholar]
- Morris, C.E.; Skalak, T.C. Chronic static magnetic field exposure alters microvessel enlargement resulting from surgical intervention. J. Appl. Physiol. 2007, 103, 629–636. [Google Scholar] [CrossRef]
- Yuksel, C.; Ankarali, S.; Yuksel, N.A. The use of neodymium magnets in healthcare and their effects on health. North. Clin. Istanb. 2018, 5, 268. [Google Scholar] [CrossRef]
- Ohkubo, C.; Okano, H. Clinical aspects of static magnetic field effects on circulatory system. Environ. 2011, 31, 97–106. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Q.; Lv, X.; Lin, T. Progressive study on the non-thermal effects of magnetic field therapy in oncology. Front. Oncol. 2021, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Colangelo, M.; Pedrazzi, G.; Guizzardi, S. The response of osteoblasts and bone to sinusoidal electromagnetic fields: Insights from the literature. Calcif. Tissue Int. 2019, 105, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Cheng, Y.; Cai, J.; Zhao, X.; Ouyang, Y.; Yuan, W.-E.; Fan, C. Advances in electrical and magnetic stimulation on nerve regeneration. Regen. Med. 2019, 14, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Azadian, E.; Arjmand, B.; Khodaii, Z.; Ardeshirylajimi, A. A comprehensive overview on utilizing electromagnetic fields in bone regenerative medicine. Electromagn. Biol. Med. 2019, 38, 1–20. [Google Scholar] [CrossRef]
- Robertson, J.A.; Thomas, A.W.; Bureau, Y.; Prato, F.S. The influence of extremely low frequency magnetic fields on cytoprotection and repair. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2007, 28, 16–30. [Google Scholar] [CrossRef]
- Moya Gomez, A.; Font, L.P.; Brône, B.; Bronckaers, A. Electromagnetic field as a treatment for cerebral ischemic stroke. Front. Mol. Biosci. 2021, 8, 742596. [Google Scholar] [CrossRef]
- Ross, C.; Harrison, B. The use of magnetic field for the reduction of inflammation: A review of the history and therapeutic results. Altern. Ther. Health Med. 2013, 19, 47–54. [Google Scholar]
- Klimek, A.; Rogalska, J. Extremely low-frequency magnetic field as a stress factor—Really detrimental?—Insight into literature from the last decade. Brain Sci. 2021, 11, 174. [Google Scholar] [CrossRef]
- Giorgi, G.; Del Re, B. Epigenetic dysregulation in various types of cells exposed to extremely low-frequency magnetic fields. Cell Tissue Res. 2021, 386, 1–15. [Google Scholar] [CrossRef]
- Nijdam, S.; Teunissen, J.; Ebert, U. The physics of streamer discharge phenomena. Plasma Sources Sci. Technol. 2020, 29, 103001. [Google Scholar] [CrossRef]
- Wu, S.; Huang, Q.; Wang, Z.; Lu, X. On the magnetic field signal radiated by an atmospheric pressure room temperature plasma jet. J. Appl. Phys. 2013, 113, 043305. [Google Scholar] [CrossRef]
- Galli, C.; Pedrazzi, G.; Mattioli-Belmonte, M.; Guizzardi, S. The use of pulsed electromagnetic fields to promote bone responses to biomaterials in vitro and in vivo. Int. J. Biomater. 2018, 2018, 8935750. [Google Scholar] [CrossRef] [PubMed]
- Pesqueira, T.; Costa-Almeida, R.; Gomes, M.E. Magnetotherapy: The quest for tendon regeneration. J. Cell. Physiol. 2018, 233, 6395–6405. [Google Scholar] [CrossRef] [PubMed]
- Shupak, N.M.; Prato, F.S.; Thomas, A.W. Therapeutic uses of pulsed magnetic-field exposure: A review. URSI Radio Sci. Bull. 2003, 2003, 9–32. [Google Scholar]
- Hu, H.; Yang, W.; Zeng, Q.; Chen, W.; Zhu, Y.; Liu, W.; Wang, S.; Wang, B.; Shao, Z.; Zhang, Y. Promising application of Pulsed Electromagnetic Fields (PEMFs) in musculoskeletal disorders. Biomed. Pharmacother. 2020, 131, 110767. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Gorbanev, Y.; Dewilde, S.; Smits, E.; Bogaerts, A. Reduction of Human Glioblastoma Spheroids Using Cold Atmospheric Plasma: The Combined Effect of Short- and Long-Lived Reactive Species. Cancers 2018, 10, 394. [Google Scholar] [CrossRef] [Green Version]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 5194780. [Google Scholar] [CrossRef]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef]
- Dubey, S.K.; Parab, S.; Alexander, A.; Agrawal, M.; Achalla, V.P.K.; Pal, U.N.; Pandey, M.M.; Kesharwani, P. Cold atmospheric plasma therapy in wound healing. Process Biochem. 2022, 112, 112–123. [Google Scholar] [CrossRef]
- Busco, G.; Robert, E.; Chettouh-Hammas, N.; Pouvesle, J.-M.; Grillon, C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic. Biol. Med. 2020, 161, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Hajipour, M.J.; Gould, L.; Mahmoudi, M. Nanomedicine in healing chronic wounds: Opportunities and challenges. Mol. Pharm. 2020, 18, 550–575. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.W.; Webster, T.J. The role of nanomedicine in growing tissues. Ann. Biomed. Eng. 2009, 37, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Wu, F.; Lu, Y.; Wei, L.; Yuan, W. Progress of electrospun fibers as nerve conduits for neural tissue repair. Nanomedicine 2014, 9, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Eslahi, N.; Ordikhani, F.; Tamjid, E.; Simchi, A. Nanomedicine applications in orthopedic medicine: State of the art. Int. J. Nanomed. 2015, 10, 6039. [Google Scholar]
- Zhou, J.; Zhang, Z.; Joseph, J.; Zhang, X.; Ferdows, B.E.; Patel, D.N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 1, 20210011. [Google Scholar] [CrossRef]
- Zinovyev, E.V.; Asadulaev, M.S.; Komissarov, I.A.; Shemet, M.V.; Yudin, V.E.; Shabunin, A.S.; Smirnova, N.V.; Kryukov, A.E.; Lukyanov, S.A.; Stoyanovskiy, R.G. Comparative assesment of efficiency application cold atmospheric plasma and biopolymerous coats evaluation of the effectiveness for the treatment of skin burns of iii degree in experiment. Pediatrics 2017, 8, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Ouf, S.A.; El-Adly, A.A.; Mohamed, A.-A.H. Inhibitory effect of silver nanoparticles mediated by atmospheric pressure air cold plasma jet against dermatophyte fungi. J. Med. Microbiol. 2015, 64, 1151–1161. [Google Scholar] [CrossRef]
- Zhu, W.; Lee, S.-J.; Castro, N.; Yan, D.; Keidar, M.; Zhang, L.G. Synergistic Effect of Cold Atmospheric Plasma and Drug Loaded Core-shell Nanoparticles on Inhibiting Breast Cancer Cell Growth OPEN. Sci. Rep. 2016, 6, 21974. [Google Scholar] [CrossRef]
- Li, W.; Yu, H.; Ding, D.; Chen, Z.; Wang, Y.; Wang, S.; Li, X.; Keidar, M.; Zhang, W. Cold atmospheric plasma and iron oxide-based magnetic nanoparticles for synergetic lung cancer therapy. Free Radic. Biol. Med. 2019, 130, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Na, K.-Y.; Lee, K.-H.; Lee, H.W.; Lee, J.K.; Kim, K.-T. Selective uptake of epidermal growth factor-conjugated gold nanoparticle (EGF-GNP) facilitates non-thermal plasma (NTP)-mediated cell death. Sci. Rep. 2017, 7, 10971. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, P.; Rehman, M.U.; Zhao, Q.-L.; Misawa, M.; Ishikawa, K.; Hori, M.; Shimizu, T.; Saitoh, J.-i.; Noguchi, K.; Kondo, T. Small size gold nanoparticles enhance apoptosis-induced by cold atmospheric plasma via depletion of intracellular GSH and modification of oxidative stress. Cell Death Discov. 2020, 6, 83. [Google Scholar] [CrossRef]
- Irani, S.; Shahmirani, Z.; Atyabi, S.M.; Mirpoor, S. Induction of growth arrest in colorectal cancer cells by cold plasma and gold nanoparticles. Arch. Med. Sci. AMS 2015, 11, 1286. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Bielawska-Pohl, A.; Pohl, P.; Jermakowicz-Bartkowiak, D.; Jamroz, P.; Malik-Gajewska, M.; Klimczak, A.; Cyganowski, P. Application of oil-in-water nanoemulsion carrying size-defined gold nanoparticles synthesized by non-thermal plasma for the human breast cancer cell lines migration and apoptosis. Plasma Chem. Plasma Process. 2020, 40, 1037–1062. [Google Scholar] [CrossRef]
- Kim, G.; Park, S.; Kim, G.; Lee, J.K.J. Targeted cancer treatment using anti-EGFR and-TFR antibody-conjugated gold nanoparticles stimulated by nonthermal air plasma. Plasma Med. 2011, 1, 45–54. [Google Scholar] [CrossRef]
- Sun, D.; McLaughlan, J.; Zhang, L.; Falzon, B.G.; Mariotti, D.; Maguire, P.; Sun, D. Atmospheric pressure plasma-synthesized gold nanoparticle/carbon nanotube hybrids for photothermal conversion. Langmuir 2019, 35, 4577–4588. [Google Scholar] [CrossRef]
- Jawaid, P.; Rehman, M.U.; Zhao, Q.L.; Takeda, K.; Ishikawa, K.; Hori, M.; Shimizu, T.; Kondo, T. Helium-based cold atmospheric plasma-induced reactive oxygen species-mediated apoptotic pathway attenuated by platinum nanoparticles. J. Cell. Mol. Med. 2016, 20, 1737–1748. [Google Scholar] [CrossRef] [Green Version]
- Gangrade, A.; Gawali, B.; Jadi, P.K.; Naidu, V.G.M.; Mandal, B.B. Photo-Electro Active Nanocomposite Silk Hydrogel for Spatiotemporal Controlled Release of Chemotherapeutics: An In Vivo Approach toward Suppressing Solid Tumor Growth. ACS Appl. Mater. Interfaces 2020, 12, 27905–27916. [Google Scholar] [CrossRef]
- Schmidt, A.; Liebelt, G.; Striesow, J.; Freund, E.; von Woedtke, T.; Wende, K.; Bekeschus, S. The molecular and physiological consequences of cold plasma treatment in murine skin and its barrier function. Free Radic. Biol. Med. 2020, 161, 32–49. [Google Scholar] [CrossRef]
- Kitamura, S.; Yamasaki, Y.; Kinomura, M.; Sugaya, T.; Sugiyama, H.; Maeshima, Y.; Makino, H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005, 19, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Fang, Y.; Zhu, L.; Al-Rubeai, M. Controlling stem cell fate using cold atmospheric plasma. Stem Cell Res. Ther. 2020, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Florini, J.R.; Ewton, D.Z.; Magri, K.A. Hormones, growth factors, and myogenic differentiation. Annu. Rev. Physiol. 1991, 53, 201–216. [Google Scholar] [CrossRef]
- Tan, F.; Rui, X.; Xiang, X.; Yu, Z.; Al-Rubeai, M. Multimodal treatment combining cold atmospheric plasma and acidic fibroblast growth factor for multi-tissue regeneration. FASEB J. 2021, 35, e21442. [Google Scholar] [CrossRef]
- Przekora, A.; Audemar, M.; Pawlat, J.; Canal, C.; Thomann, J.-S.; Labay, C.; Wojcik, M.; Kwiatkowski, M.; Terebun, P.; Ginalska, G.; et al. Positive Effect of Cold Atmospheric Nitrogen Plasma on the Behavior of Mesenchymal Stem Cells Cultured on a Bone Scaffold Containing Iron Oxide-Loaded Silica Nanoparticles Catalyst. Int. J. Mol. Sci. 2020, 21, 4738. [Google Scholar] [CrossRef] [PubMed]
- Barnsley, G.P.; Sigurdson, L.J.; Barnsley, S.E. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: A meta-analysis of randomized controlled trials. Plast. Reconstr. Surg. 2006, 117, 2182–2190. [Google Scholar] [CrossRef]
- Brohim, R.M.; Foresman, P.A.; Hildebrandt, P.K.; Rodeheaver, G.T. Early tissue reaction to textured breast implant surfaces. Ann. Plast. Surg. 1992, 28, 354–362. [Google Scholar] [CrossRef]
- Hauser, J.; Zietlow, J.; Köller, M.; Esenwein, S.A.; Halfmann, H.; Awakowicz, P.; Steinau, H.U. Enhanced cell adhesion to silicone implant material through plasma surface modification. J. Mater. Sci. Mater. Med. 2009, 20, 2541. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.B. Platelet adhesion onto wettability gradient surfaces in the absence and presence of plasma proteins. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 41, 304–311. [Google Scholar] [CrossRef]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Ueda, Y.; Fujita, S.; Nishigaki, T.; Arima, Y.; Iwata, H. Substrates for human pluripotent stem cell cultures in conditioned medium of mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2012, 23, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kulangara, K.; Lam, R.T.S.; Dharmawan, R.; Leong, K.W. Effects of Topographical and Mechanical Property Alterations Induced by Oxygen Plasma Modification on Stem Cell Behavior. ACS Nano 2012, 6, 8591–8598. [Google Scholar] [CrossRef]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Progress and promise of nitric oxide-releasing platforms. Adv. Sci. 2018, 5, 1701043. [Google Scholar] [CrossRef]
- Pinto, R.V.; Carvalho, S.; Antunes, F.; Pires, J.; Pinto, M.L. Emerging Nitric Oxide and Hydrogen Sulfide Releasing Carriers for Skin Wound Healing Therapy. ChemMedChem 2022, 17, e202100429. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Wu, M.; Lu, Z.; Wu, K.; Nam, C.; Zhang, L.; Guo, J. Recent advances in the development of nitric oxide-releasing biomaterials and their application potentials in chronic wound healing. J. Mater. Chem. B 2021, 9, 7063–7075. [Google Scholar] [CrossRef] [PubMed]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.; Bogaerts, A. Correction: Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2016, 116, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogaerts, A.; Tu, X.; Whitehead, J.C.; Centi, G.; Lefferts, L.; Guaitella, O.; Azzolina-Jury, F.; Kim, H.-H.; Murphy, A.B.; Schneider, W.F.; et al. The 2020 plasma catalysis roadmap. J. Phys. D Appl. Phys. 2020, 53, 443001. [Google Scholar] [CrossRef]
- Bogaerts, A.; Neyts, E.C.; Guaitella, O.; Murphy, A.B. Foundations of plasma catalysis for environmental applications. Plasma Sources Sci. Technol. 2022, 31, 053002. [Google Scholar] [CrossRef]
- Olatunde, O.C.; Onwudiwe, D.C. Graphene-Based Composites as Catalysts for the Degradation of Pharmaceuticals. Int. J. Environ. Res. Public Health 2021, 18, 1529. [Google Scholar] [CrossRef]
- Russo, M.; Iervolino, G.; Vaiano, V.; Palma, V. Non-Thermal Plasma Coupled with Catalyst for the Degradation of Water Pollutants: A Review. Catalysts 2020, 10, 1438. [Google Scholar] [CrossRef]
- Kumar, A.; Škoro, N.; Gernjak, W.; Puač, N. Cold atmospheric plasma technology for removal of organic micropollutants from wastewater—A review. Eur. Phys. J. D 2021, 75, 283. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef]
- Zhang, Z.; Klausen, L.H.; Chen, M.; Dong, M. Electroactive Scaffolds for Neurogenesis and Myogenesis: Graphene-Based Nanomaterials. Small 2018, 14, 1801983. [Google Scholar] [CrossRef]
- Yao, X.; Qian, Y.; Fan, C. Electroactive nanomaterials in the peripheral nerve regeneration. J. Mater. Chem. B 2021, 9, 6958–6972. [Google Scholar] [CrossRef]
- Hu, N.; Ai, Y.; Qian, S. Field effect control of electrokinetic transport in micro/nanofluidics. Sens. Actuators B Chem. 2012, 161, 1150–1167. [Google Scholar] [CrossRef]
- Gomes, H.I.; Rodríguez-Maroto, J.M.; Ribeiro, A.B.; Pamukcu, S.; Dias-Ferreira, C. Electrokinetics and zero valent iron nanoparticles: Experimental and modeling of the transport in different porous media. In Electrokinetics across Disciplines and Continents; Springer: Cham, Switzerland, 2016; pp. 279–294. [Google Scholar]
- Kaushik, A.; Jayant, R.D.; Sagar, V.; Nair, M. The potential of magneto-electric nanocarriers for drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Shen, Z.; Yu, L.; Wei, M.; Li, Y. Manipulating nanoparticle transport within blood flow through external forces: An exemplar of mechanics in nanomedicine. Proc. R. Soc. A Math. Phys. Eng. Sci. 2018, 474, 20170845. [Google Scholar] [CrossRef]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, X.; Wang, D.; Wang, S.; Liu, Z.; Zhao, G.; Zhang, Y.; Li, Z.; Wang, Z.L.; Li, L. Piezoelectric nanotopography induced neuron-like differentiation of stem cells. Adv. Funct. Mater. 2019, 29, 1900372. [Google Scholar] [CrossRef]
- Khare, D.; Basu, B.; Dubey, A. Electrically stimulated piezoelectric biomaterials as next generation implants for orthopedic applications. Biomaterials 2020, 258, 120280. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.; Mano, J.F. Magnetic composite biomaterials for tissue engineering. Biomater. Sci. 2014, 2, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Xue, J.; Deb, S. Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials 2022, 12, 757. [Google Scholar] [CrossRef] [PubMed]
- Markides, H.; Rotherham, M.; El Haj, A. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 2012, 13. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013, 42, 5552–5576. [Google Scholar] [CrossRef]
- Saifi, M.A.; Khan, W.; Godugu, C. Cytotoxicity of nanomaterials: Using nanotoxicology to address the safety concerns of nanoparticles. Pharm. Nanotechnol. 2018, 6, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Elsaesser, A.; Howard, C.V. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; Pillai, B.; Das, T.K.; Singh, Y.; Maiti, S. Molecular effects of uptake of gold nanoparticles in HeLa cells. ChemBioChem 2007, 8, 1237–1240. [Google Scholar] [CrossRef]
- Duan, J.; Lu, X.; He, G. On the penetration depth of reactive oxygen and nitrogen species generated by a plasma jet through real biological tissue. Phys. Plasmas 2017, 24, 073506. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Shashurin, A.; Keidar, M.; Bronnikov, S.; Jurjus, R.A.; Stepp, M.A. Living tissue under treatment of cold plasma atmospheric jet. Appl. Phys. Lett. 2008, 93, 181501. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef]
- Boehm, D.; Bourke, P. Safety implications of plasma-induced effects in living cells—A review of in vitro and in vivo findings. Biol. Chem. 2019, 400, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, P.; Vanraes, P.; Kumar, N.; Bogaerts, A. Possible Synergies of Nanomaterial-Assisted Tissue Regeneration in Plasma Medicine: Mechanisms and Safety Concerns. Nanomaterials 2022, 12, 3397. https://doi.org/10.3390/nano12193397
Shaw P, Vanraes P, Kumar N, Bogaerts A. Possible Synergies of Nanomaterial-Assisted Tissue Regeneration in Plasma Medicine: Mechanisms and Safety Concerns. Nanomaterials. 2022; 12(19):3397. https://doi.org/10.3390/nano12193397
Chicago/Turabian StyleShaw, Priyanka, Patrick Vanraes, Naresh Kumar, and Annemie Bogaerts. 2022. "Possible Synergies of Nanomaterial-Assisted Tissue Regeneration in Plasma Medicine: Mechanisms and Safety Concerns" Nanomaterials 12, no. 19: 3397. https://doi.org/10.3390/nano12193397