Electrochemical Performance of Chemically Activated Carbons from Sawdust as Supercapacitor Electrodes
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Chemicals
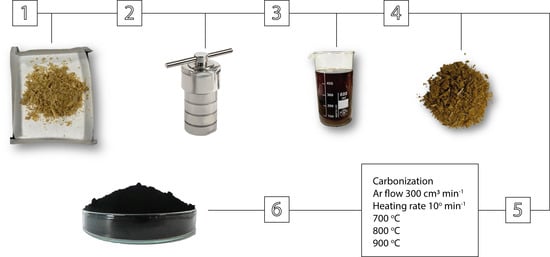
2.2. Preparation of Activated Carbon
2.3. Characterization
2.4. Electrochemical Measurements
2.4.1. Preparation of Electrodes
2.4.2. Electrochemical Measurements
3. Results and Discussion
3.1. Activated Carbon Surface Characterization
3.2. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Liang, Y.; Hu, H.; Liu, S.; Cai, Y.; Dong, H.; Zheng, M.; Xiao, Y.; Liu, Y. Ultrahigh-surface-area hierarchical porous carbon from chitosan: Acetic acid mediated efficient synthesis and its application in superior supercapacitors. J. Mater. Chem. A 2017, 5, 24775–24781. [Google Scholar] [CrossRef]
- Xie, K.; Qin, X.; Wang, X.; Wang, Y.; Tao, H.; Wu, Q.; Yang, L.; Hu, Z. Carbon nanocages as supercapacitor electrode materials. Adv. Mater. 2012, 24, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Peng, X.; Zhang, X.; Jing, S.; Zhong, L.; Sun, R. Facile synthesis of cellulose-based carbon with tunable N content for potential supercapacitor application. Carbohydr. Polym. 2017, 170, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, L.; Shi, T.; Zha, R. Nanocasting and direct synthesis strategies for mesoporous carbons as supercapacitor electrodes. Chem. Mater. 2018, 30, 7391–7412. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, F.; Cheong, J.Y.; Lee, J.; Ahn, J.; Duan, G.; Chen, H.; Zhang, Q.; Kim, I.-D. Pyrolysis of enzymolysis-treated wood: Hierarchically assembled porous carbon electrode for advanced energy storage devices. Adv. Funct. Mater. 2021, 31, 2101077–21010786. [Google Scholar]
- Borchardt, L.; Zhu, Q.; Casco, M.E.; Berger, R.; Zhuang, X.; Kaskel, S.; Feng, X.; Xu, Q. Toward a molecular design of porous carbon materials. Mater. Today 2017, 20, 592–610. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 25. [Google Scholar] [CrossRef]
- Wang, Y.M.; Lin, X.; Liu, T.; Chen, H.; Chen, S.; Jiang, Z.; Liu, J.; Huang, J.; Liu, M. Wood-derived hierarchically porous electrodes for high-performance all-solid-state supercapacitors. Adv. Funct. Mater. 2018, 28, 1806207. [Google Scholar] [CrossRef]
- Long, Y.; An, X.; Zhang, H.; Yang, J.; Liu, L.; Tian, Z.; Yang, G.; Cheng, Z.; Cao, H.; Liu, H.; et al. Highly graphitized lignin-derived porous carbon with hierarchical N/O co-doping “core-shell” superstructure supported by metal-organic frameworks for advanced supercapacitor performance. Chem. Eng. J. 2022, 451, 138877. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Du, C.; Jian, S.; Yang, W.; Wu, Y.A.; Jiang, S.; He, S.; Chen, W. Wood-derived biochar as thick electrodes for high-rate performance supercapacitors. Biochar 2022, 4, 50. [Google Scholar] [CrossRef]
- Mapleback, B.; Dao, V.; Webb, L.; Rider, A. Composite Structural Supercapacitors: High-Performance Carbon Nanotube Supercapacitors through Ionic Liquid Localisation. Nanomaterials 2022, 12, 2558. [Google Scholar] [CrossRef] [PubMed]
- Sarangapani, S.; Tilak, B.; Chen, C.P. Materials for electrochemical capacitors: Theoretical and experimental constraints. J. Electrochem. Soc. 1996, 143, 3791. [Google Scholar] [CrossRef]
- Fic, K.; Platek, A.; Piwek, J.; Frackowiak, E. Sustainable materials for electrochemical capacitors. Mater. Today 2018, 21, 437–454. [Google Scholar] [CrossRef]
- Elsaidy, A.; Majcherkiewicz, J.N.; Puértolas, B.; Salgueiriño, V.; Nóvoa, X.R.; Correa-Duarte, M.A. Synergistic Interaction of Clusters of Iron Oxide Nanoparticles and Reduced Graphene Oxide for High Supercapacitor Performance. Nanomaterials 2022, 12, 2695. [Google Scholar] [CrossRef]
- Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Ramachandra, B.; Sahajwalla, V. Performance of an activated carbon supercapacitor electrode synthesised from waste Compact Discs (CDs). J. Ind. Eng. Chem. 2018, 65, 387–396. [Google Scholar] [CrossRef]
- Timperman, L.; Vigeant, A.; Anouti, M. Eutectic mixture of protic ionic liquids as an electrolyte for activated carbon-based supercapacitors. Electrochim. Acta 2015, 155, 164–173. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Qin, F.; Yuan, J.; Zhang, K.; Li, J.; Zhud, D.-M.; Qine, L.-C. Hybrid lithium-ion capacitors with asymmetric graphene electrodes. J. Mater. Chem. A 2017, 5, 13601–13609. [Google Scholar] [CrossRef]
- Kshetri, T.; Thanh, T.; Singh, S.; Kim, N.; Lee, J. Hierarchical material of carbon nanotubes grown on carbon nanofibers for high performance electrochemical capacitor. Chem. Eng. J. 2018, 345, 39–47. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Wang, L.; Xu, H.; Zhong, J.; Zhang, M.; Wang, Y.; Zhang, Q.; Mei, L.; Wang, T.; et al. Nitrogen-doped carbon nanotubes as an anode for a highly robust potassium-ion hybrid capacitor. Nanoscale Horiz. 2020, 5, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Kwac, L.; An, K.; Park, C.; Kim, B. Electrochemical behavior of pitch-based activated carbon fibers for electrochemical capacitors. Energy Convers. Manag. 2016, 125, 347–352. [Google Scholar] [CrossRef]
- Yuan, W.; Han, G.; Xiao, Y.; Chang, Y.; Liu, C.; Li, M.; Li, Y.; Zhang, Y. Flexible electrochemical capacitors based on polypyrrole/carbon fibers via chemical polymerization of pyrrole vapor. Appl. Surf. Sci. 2016, 377, 274–282. [Google Scholar] [CrossRef]
- Zhou, X.; Tong, W.; Huang, F.; Chen, L.; Wu, H. Methylene blue enhanced bamboo activated carbon as high performance supercapacitor electrode materials. Ind. Crops Prod. 2022, 180, 114786. [Google Scholar]
- Özsin, G.; Kılıç, M.; Apaydın-Varol, E.; Pütün, A. Chemically activated carbon production from agricultural waste of chickpea and its application for heavy metal adsorption: Equilibrium, kinetic, and thermodynamic studies. Appl. Water Sci. 2019, 9, 56. [Google Scholar] [CrossRef]
- Fasakin, O.; Dangbegnon, J.K.; Momodu, D.Y.; Madito, M.J.; Oyedotun, K.O.; Eleruja, M.A.; Manyala, N. Synthesis and characterization of porous carbon derived from activated banana peels with hierarchical porosity for improved electrochemical performance. Electrochim. Acta 2018, 262, 187–196. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Badhulika, S. Ultrathin Graphene-like 2D porous carbon nanosheets and its excellent capacitance retention for supercapacitor. J. Ind. Eng. Chem. 2018, 68, 257–266. [Google Scholar] [CrossRef]
- Hoffman, V.; Jung, D.; Alhnidi, J.A.; Mackle, L.; Kruse, A. Bio-Based Carbon Materials from Potato Waste as Electrode Materials in Supercapacitors. Energies 2020, 13, 2406. [Google Scholar] [CrossRef]
- Gunes, A.Y.; Changle, J.; Yumak, T.; Zondlo, J.; Wang, J.; Sabolsky, E. Engineered hierarchical porous carbons for supercapacitor applications through chemical pretreatment and activation of biomass precursors. Renew. Energy 2021, 163, 276–287. [Google Scholar]
- Shrestha, D. Activated carbon and its hybrid composites with manganese (IV) oxide as effectual electrode materials for high performance supercapacitor. Arab. J. Chem. 2022, 15, 103946. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, B.; Feng, L.; Zhang, Q. Potassium citrate assisted synthesis of hierarchical porous carbon materials for high performance supercapacitors. Diam. Relat. Mater. 2022, 128, 109247. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.; Luque, R.; Clark, J.; Macquarrie, D. Tuneable porous carbonaceous materials from renewable resources. Chem. Soc. Rev. 2009, 38, 3401–3418. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Gu, D.; Zou, Y.; Wu, Z. A low-concentration hydrothermal synthesis of biocompatible ordered mesoporous carbon nanospheres with tunable and uniform size. Angew. Chem. Int. Ed. 2010, 49, 7987–7991. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Lu, L.; Luo, Z.; Yang, B.; Ke, J.; Lei, W.; Zhen, H.; Zhuang, Y.; Sun, J.; Chen, K.; et al. Heteroatom-Doped Hierarchically Porous Biochar for Supercapacitor Application and Phenol Pollutant Remediation. Nanomaterials 2022, 12, 2586. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, S.A.; Alqadami, A.A.; Khan, M.A.; Alanazi, H.S.; Siddiqui, M.R.; Jeon, B.-H. Simultaneous co-hydrothermal carbonization and chemical activation of food wastes to develop hydrochar for aquatic environmental remediation. Bioresour. Technol. 2022, 347, 126363. [Google Scholar] [CrossRef]
- Diaz, E.; Sanchis, I.; Coronella, C.; Mohedano, A.F. Activated Carbons from Hydrothermal Carbonization and Chemical Activation of Olive Stones: Application in Sulfamethoxazole Adsorption. Resources 2022, 11, 43. [Google Scholar] [CrossRef]
- Suhdi, S.; Wang, S.-C. The Production of Carbon Nanofiber on Rubber Fruit Shell-Derived Activated Carbon by Chemical Activation and Hydrothermal Process with Low Temperature. Nanomaterials 2021, 11, 2038. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Yerlanuly, Y.; Zhumadilov, R.; Nemkayeva, R.; Uzakbaiuly, B.; Beisenbayev, A.R.; Bakenov, Z.; Ramazanov, T.; Gabdullin, M.; Ng, A.; Brus, V.; et al. Physical properties of carbon nanowalls synthesized by the ICP-PECVD method vs. the growth time. Sci. Rep. 2021, 11, 19287. [Google Scholar] [CrossRef]
- Chengkai, X.; Subramani, S.; Seulgi, J.; Kim, D. A sulfur self-doped multifunctional biochar catalyst for overall water splitting and a supercapacitor from Camellia japonica flowers. Carbon Energy 2022, 4, 491–505. [Google Scholar]
- Ahmed, S.; Ahmed, A.; Rafat, M. Investigation on activated carbon derived from biomass Butnea monosperma and its application as a high performance supercapacitor electrode. J. Energy Storage 2019, 26, 100988. [Google Scholar] [CrossRef]
- Cao, L.; Li, H.; Xu, Z.; Gao, R.; Wang, S.; Zhang, G.; Jiang, S.; Xu, W.; Hou, H. Camellia pollen-derived carbon with controllable N content for high-performance supercapacitors by ammonium chloride activation and dual N-doping. Chem. Nano. Mat. 2021, 7, 34–43. [Google Scholar] [CrossRef]








| Sample | AKS-700 | AKS-800 | AKS-900 | APS-700 | APS-800 | APS-900 |
|---|---|---|---|---|---|---|
| SBET, (m2 g−1) | 505 | 557 | 532 | 494 | 715 | 666 |
| C | O | K | Ca | Fe | Ni | |
|---|---|---|---|---|---|---|
| AKS-700 | 90.29 | 7.30 | 1.80 | 0.57 | − | − |
| AKS-800 | 85.37 | 14.63 | − | − | 1.80 | 0.57 |
| AKS-900 | 84.69 | 10.90 | 1.17 | − | 1.41 | 1.83 |
| APS-700 | 87.14 | 12.86 | − | − | − | − |
| APS-800 | 93.87 | 6.13 | − | − | − | − |
| APS-900 | 87.065 | 12.95 | − | − | − | − |
| Samples | Scan Rate, mV s−1 | |||||
|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 40 | 80 | 160 | |
| Specific Capacitance, F g−1 | ||||||
| AKS-700 | 202 | 194 | 185 | 175 | 162 | 147 |
| AKS-800 | 128 | 111 | 91 | 65 | 42 | 28 |
| AKS-900 | 121 | 113 | 105 | 102 | 99 | 92 |
| APS-700 | 161 | 155 | 148 | 139 | 127 | 114 |
| APS-800 | 146 | 142 | 135 | 126 | 115 | 104 |
| APS-900 | 115 | 108 | 99 | 87 | 73 | 59 |
| Samples | Current Density, mA g−1 | ||||
|---|---|---|---|---|---|
| 2000 | 1000 | 500 | 250 | 100 | |
| Specific Capacitance, F g−1 | |||||
| AKS-700 | 178 | 183 | 187 | 189 | 193 |
| AKS-800 | 106 | 111 | 117 | 131 | 148 |
| AKS-900 | 102 | 104 | 105 | 106 | 104 |
| APS-700 | 131 | 140 | 147 | 153 | 159 |
| APS-800 | 109 | 115 | 120 | 123 | 126 |
| APS-900 | 91 | 104 | 112 | 118 | 118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazhipkyzy, M.; Yeleuov, M.; Sultakhan, S.T.; Maltay, A.B.; Zhaparova, A.A.; Assylkhanova, D.D.; Nemkayeva, R.R. Electrochemical Performance of Chemically Activated Carbons from Sawdust as Supercapacitor Electrodes. Nanomaterials 2022, 12, 3391. https://doi.org/10.3390/nano12193391
Nazhipkyzy M, Yeleuov M, Sultakhan ST, Maltay AB, Zhaparova AA, Assylkhanova DD, Nemkayeva RR. Electrochemical Performance of Chemically Activated Carbons from Sawdust as Supercapacitor Electrodes. Nanomaterials. 2022; 12(19):3391. https://doi.org/10.3390/nano12193391
Chicago/Turabian StyleNazhipkyzy, Meruyert, Mukhtar Yeleuov, Shynggyskhan T. Sultakhan, Anar B. Maltay, Aizhan A. Zhaparova, Dana D. Assylkhanova, and Renata R. Nemkayeva. 2022. "Electrochemical Performance of Chemically Activated Carbons from Sawdust as Supercapacitor Electrodes" Nanomaterials 12, no. 19: 3391. https://doi.org/10.3390/nano12193391