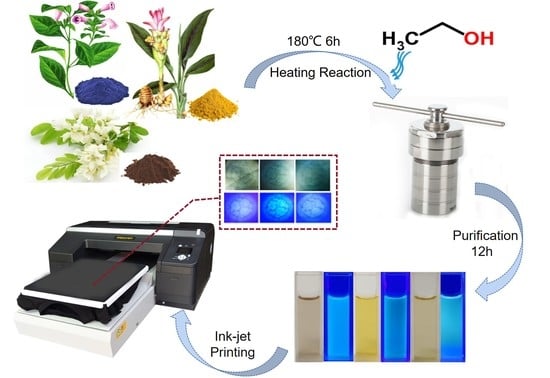
Dye Plants Derived Carbon Dots for Flexible Secure Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of C-Dots
2.3. Ink Preparation and Printing
2.4. Characterizations
3. Results and Discussion
3.1. Synthesis and Structure of C-dots
3.2. Optical Properties of C-Dots
3.3. Optical Anti-Counterfeiting System
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Shen, D.; Wang, Q.; Luo, K.H. Green synthesis of tunable fluorescent carbon quantum dots from lignin and their application in anti-counterfeit printing. ACS Appl. Mater. Interfaces 2021, 13, 56465–56475. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Li, J.; Liu, G.; Gao, M.; Ren, S.; Liu, B.; Zhang, L.; Han, G.; Yu, J.; et al. Platinum cluster/carbon quantum dots derived graphene heterostructured carbon nanofibers for efficient and durable solar-driven electrochemical hydrogen evolution. Small Methods 2022, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, W.; Dong, H.; Wei, M. Graphene quantum dots for optical bioimaging. Small 2019, 15, 1–19. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Wang, H.-Q.; Yang, Q.; Yu, J.-H.; Zhao, Y.-D. Hydrogen peroxide biosensor based on direct electron transfer of horseradish peroxidase with vapor deposited quantum dots. Sens. Actuators B 2009, 138, 278–282. [Google Scholar] [CrossRef]
- Safardoust-Hojaghan, H.; Salavati-Niasari, M. Degradation of methylene blue as a pollutant with N-doped graphene quantum dot/titanium dioxide nanocomposite. J. Clean. Prod. 2017, 148, 31–36. [Google Scholar] [CrossRef]
- Xu, L.; Bai, X.; Guo, L.; Yang, S.; Jin, P.; Yang, L. Facial fabrication of carbon quantum dots (CDs)-modified N-TiO2−x nanocomposite for the efficient photoreduction of Cr(VI) under visible light. Chem. Eng. J. 2019, 357, 473–486. [Google Scholar] [CrossRef]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining carbon dots: Synthesis and biomedical and optoelectronic applications. Nano Today 2016, 11, 565–586. [Google Scholar]
- Zhao, B.; Tan, Z. Fluorescent carbon dots: Fantastic electroluminescent materials for light-emitting diodes. Adv. Sci. 2021, 8, 1–20. [Google Scholar]
- Liu, Y.; Zhou, L.; Li, Y.; Deng, R.; Zhang, H. Highly fluorescent nitrogen-doped carbon dots with excellent thermal and photo stability applied as invisible ink for loading important information and anti-counterfeiting. Nanoscale 2017, 9, 491–496. [Google Scholar] [CrossRef]
- Song, Z.; Lin, T.; Lin, L.; Lin, S.; Fu, F.; Wang, X.; Guo, L. Invisible security ink based on water-soluble graphitic carbon nitride quantum dots. Angew. Chem. Int. Ed. 2016, 55, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Kalytchuk, S.; Wang, Y.; Polakova, K.; Zboril, R. Carbon dot fluorescence-lifetime-encoded anti-counterfeiting. ACS Appl. Mater. Interfaces 2018, 10, 29902–29908. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Cheng, R.; Ji, W.Q.; Ma, K.; Ling, L.; Chen, S. Recognition of latent fingerprints and ink-free printing derived from interfacial segregation of carbon dots. ACS Appl. Mater. Interfaces 2018, 10, 39205–39213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhong, S.; Wu, J.; Geng, Y.; Zhou, B.; Wang, Q.; Jiang, W. Facile preparation and the stepwise formation mechanistic investigation of gram-scale nitrogen-doped graphene quantum dots. J. Mater. Chem. C 2017, 5, 9174–9180. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, F.; Yu, J.; Deng, Q.; Zhang, F.; Wang, G. Titanium carbide (Ti3C2Tx) MXene: A novel precursor to amphiphilic carbide-derived graphene quantum dots for fluorescent ink, light-emitting composite and bioimaging. Carbon 2017, 118, 50–57. [Google Scholar] [CrossRef]
- Dehnavi, E.; Shams-Nateri, A.; Khalili, H. Wool dyeing with binary mixture of natural dyes. Pigment Resin Technol. 2016, 45, 52–61. [Google Scholar] [CrossRef]
- Richhariya, G.; Kumar, A.; Tekasakul, P.; Gupta, B. Natural dyes for dye sensitized solar cell: A review. Renew. Sustain. Energy Rev. 2017, 69, 705–718. [Google Scholar] [CrossRef]
- Costa, A.F.S.; de Amorim, J.D.P.; Almeida, F.C.G.; de Lima, I.D.; de Paiva, S.C.; Rocha, M.A.V.; Vinhas, G.M.; Sarubbo, L.A. Dyeing of bacterial cellulose films using plant-based natural dyes. Int. J. Biol. Macromol. 2019, 121, 580–587. [Google Scholar] [CrossRef]
- Santos, C.; Brum, L.F.W.; de Fátima Vasconcelos, R.; Velho, S.K.; dos Santos, J.H.Z. Color and fastness of natural dyes encapsulated by a sol-gel process for dyeing natural and synthetic fibers. J. Sol-Gel Sci. Technol. 2018, 86, 351–364. [Google Scholar] [CrossRef]
- Shams Nateri, A.; Dehnavi, E.; Hajipour, A.; Ekrami, E. Dyeing of polyamide fibre with cochineal natural dye. Pigment Resin Technol. 2016, 45, 252–258. [Google Scholar] [CrossRef]
- Haji, A.; Naebe, M. Cleaner dyeing of textiles using plasma treatment and natural dyes: A review. J. Clean. Prod. 2020, 265, 1–10. [Google Scholar]
- Tang, L.; Ji, R.; Li, X.; Bai, G.; Liu, C.P.; Hao, J.; Lin, J.; Jiang, H.; Teng, K.S.; Yang, Z.; et al. Deep ultraviolet to near-infrared emission and photoresponse in layered N-doped graphene quantum dots. ACS Nano 2014, 8, 6312–6320. [Google Scholar] [CrossRef]
- Saikhao, L.; Setthayanond, J.; Karpkird, T.; Bechtold, T.; Suwanruji, P. Green reducing agents for indigo dyeing on cotton fabrics. J. Clean. Prod. 2018, 197, 106–113. [Google Scholar] [CrossRef]
- Zsila, F.; Bikádi, Z.; Simonyi, M. Molecular basis of the Cotton effects induced by the binding of curcumin to human serum albumin. Tetrahedron Asymmetry 2003, 14, 2433–2444. [Google Scholar] [CrossRef]
- Horosanskaia, E.; Minh Nguyen, T.; Dinh Vu, T.; Seidel-Morgenstern, A.; Lorenz, H. Crystallization-based isolation of pure rutin from herbal extract of Sophora japonica L. Org. Process Res. Dev. 2017, 21, 1769–1778. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar]
- Zheng, M.; Liu, S.; Li, J.; Qu, D.; Zhao, H.; Guan, X.; Hu, X.; Xie, Z.; Jing, X.; Sun, Z. Integrating oxaliplatin with highly luminescent carbon dots: An unprecedented theranostic agent for personalized medicine. Adv. Mater. 2014, 26, 3554–3560. [Google Scholar] [CrossRef]
- Zhou, D.; Jing, P.; Wang, Y.; Zhai, Y.; Li, D.; Xiong, Y.; Baranov, A.V.; Qu, S.; Rogach, A.L. Carbon dots produced via space-confined vacuum heating: Maintaining efficient luminescence in both dispersed and aggregated states. Nanoscale Horiz. 2019, 4, 388–395. [Google Scholar]
- Zhu, W.; Feng, X.Q.; Zhao, M.H.; Wei, Z.H.; Liu, Z.D.; Wang, G.; Guo, Q.L.; Chen, D. Scalable and atom economic preparation of red-near-infrared emitted N-doped graphene quantum dots with a high quantum yield. Diam. Relat. Mater. 2021, 116, 1–7. [Google Scholar] [CrossRef]
- Hsu, P.C.; Shih, Z.Y.; Lee, C.H.; Chang, H.T. Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem. 2012, 14, 917–920. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Zhai, J.F.; Dong, S.J. Bifunctional fluorescent carbon nanodots: Green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem. Commun. 2012, 48, 9367–9369. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Z.; Li, H.; Ling, L.T.; Li, G.; Cheng, R.; Lu, X.; Xie, A.Q.; Li, Q.; Wang, C.F.; Chen, S. Green synthesis of carbon dots toward anti-counterfeiting. ACS Sustain. Chem. Eng. 2019, 8, 1566–1572. [Google Scholar] [CrossRef]
- Ren, S.; Liu, B.; Wang, M.; Han, G.; Zhao, H.; Zhang, Y. Highly bright carbon quantum dots for flexible anti-counterfeiting. J. Mater. Chem. C 2022, 10, 11338–11346. [Google Scholar] [CrossRef]





| Raw Material | Major Component | Abs (nm) | PL Peak (nm) | Τ1 (ns) | Τ2 (ns) | Average Lifetime (ns) | QY (%) |
|---|---|---|---|---|---|---|---|
 |  | 220–450 | 400 | 1.6 | 8.7 | 3.0 | 3.8 |
 |  | 220–450 | 460 | 0.3 | 5.2 | 3.9 | 0.8 |
 |  | 220–450 | 420 | 0.9 | 4.5 | 1.0 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Han, Y.; Wang, L.; Jiang, W.; Zhao, H. Dye Plants Derived Carbon Dots for Flexible Secure Printing. Nanomaterials 2022, 12, 3168. https://doi.org/10.3390/nano12183168
Li L, Han Y, Wang L, Jiang W, Zhao H. Dye Plants Derived Carbon Dots for Flexible Secure Printing. Nanomaterials. 2022; 12(18):3168. https://doi.org/10.3390/nano12183168
Chicago/Turabian StyleLi, Linlin, Yuanyuan Han, Lihua Wang, Wei Jiang, and Haiguang Zhao. 2022. "Dye Plants Derived Carbon Dots for Flexible Secure Printing" Nanomaterials 12, no. 18: 3168. https://doi.org/10.3390/nano12183168