Construction of Prochloraz-Loaded Hollow Mesoporous Silica Nanoparticles Coated with Metal–Phenolic Networks for Precise Release and Improved Biosafety of Pesticides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
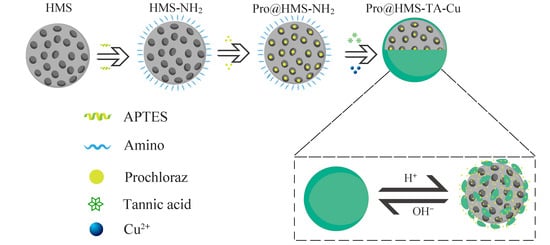
2.2. Synthesis of Pro@HMS-TA-Cu Nanoparticles
2.2.1. Preparation of HMS
2.2.2. Preparation of HMS-NH2
2.2.3. Preparation of HMS-TA-Cu
2.2.4. Prochloraz Loading
2.3. Characterization
2.4. Controlled Release Characteristics
2.5. Determination of Adhesion Properties
2.6. In Vitro Antifungal Studies of Pro@HMS-TA-Cu
2.7. Toxicity of Pro@HMS-TA-Cu to Zebrafish
3. Results and Discussion
3.1. Preparation and Characterization of HMS-TA-Cu
3.2. Pesticide Loading and Controlled Release Behavior
3.3. Determination of Adhesion Properties
3.4. Antifungal Activity of Pro@HMS-TA-Cu against S. sclerotiorum
3.5. Safety Evaluation of Pro@HMS-TA-Cu to Adult Zebrafish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Gao, Y.; Liu, Y.; Huang, X.; Zhang, D.X.; Cao, H.; Jing, T.; Liu, F.; Li, B. Self-Assembled Degradable Nanogels Provide Foliar Affinity and Pinning for Pesticide Delivery by Flexibility and Adhesiveness Adjustment. ACS Nano 2021, 15, 14598–14609. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhou, Z.; Niu, S.; Cao, C.; Li, X.; Shan, Y.; Huang, Q. Positive-Charge Functionalized Mesoporous Silica Nanoparticles as Nanocarriers for Controlled 2,4-Dichlorophenoxy Acetic Acid Sodium Salt Release. J. Agric. Food Chem. 2018, 66, 6594–6603. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, Y.; Fan, C.; Dong, H.; Yang, J.; Tang, J.; Tang, G.; Wang, W.; Jiang, N.; Cao, Y. Preparation of kasugamycin conjugation based on ZnO quantum dots for improving its effective utilization. Chem. Eng. J. 2019, 361, 671–679. [Google Scholar] [CrossRef]
- Song, S.; Wang, Y.; Xie, J.; Sun, B.; Zhou, N.; Shen, H.; Shen, J. Carboxymethyl Chitosan Modified Carbon Nanoparticle for Controlled Emamectin Benzoate Delivery: Improved Solubility, pH-Responsive Release, and Sustainable Pest Control. ACS Appl. Mater. Interfaces 2019, 11, 34258–34267. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Fan, C.; Dong, H.; Zhang, W.; Tang, G.; Yang, J.; Jiang, N.; Cao, Y. Preparation of MSNs-Chitosan@Prochloraz Nanoparticles for Reducing Toxicity and Improving Release Properties of Prochloraz. ACS Sustain. Chem. Eng. 2018, 6, 10211–10220. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, C.; Zhang, S.; Zheng, L.; Li, F.; Cao, C.; Cao, L.; Huang, Q. Fungicide-loaded mesoporous silica nanoparticles promote rice seedling growth by regulating amino acid metabolic pathways. J. Hazard. Mater. 2022, 425, 127892. [Google Scholar] [CrossRef]
- Feng, P.; Chen, J.; Fan, C.; Huang, G.; Yu, Y.; Wu, J.; Lin, B. An eco-friendly MIL-101@CMCS double-coated dinotefuran for long-acting active release and sustainable pest control. J. Clean. Prod. 2020, 265, 121851. [Google Scholar] [CrossRef]
- Dong, J.; Chen, W.; Feng, J.; Liu, X.; Xu, Y.; Wang, C.; Yang, W.; Du, X. Facile, Smart, and Degradable Metal-Organic Framework Nanopesticides Gated with Fe(III)-Tannic Acid Networks in Response to Seven Biological and Environmental Stimuli. ACS Appl. Mater. Interfaces 2021, 13, 19507–19520. [Google Scholar] [CrossRef]
- Gao, Y.; Kaziem, A.E.; Zhang, Y.; Xiao, Y.; He, S.; Li, J. A hollow mesoporous silica and poly(diacetone acrylamide) composite with sustained-release and adhesion properties. Microporous Mesoporous Mater. 2018, 255, 15–22. [Google Scholar] [CrossRef]
- Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kaushik, A.; Sonne, C.; Kim, K.H.; Kumar, S. Emerging nanobiotechnology in agriculture for the management of pesticide residues. J. Hazard. Mater. 2021, 401, 123369. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, Q.; Peng, M.; Zhou, Z.; Du, X.; Yin, M.; Shen, J.; Yan, S. A Star Polyamine-Based Nanocarrier Delivery System for Enhanced Avermectin Contact and Stomach Toxicity against Green Peach Aphids. Nanomaterials 2022, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Feng, J.; He, K.; Chen, Z.; Chen, W.; Cao, H.; Yuan, S. Preparation of thermosensitive buprofezin-loaded mesoporous silica nanoparticles by the sol-gel method and their application in pest control. Pest Manag. Sci. 2021, 77, 4627–4637. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; He, S.; Xiao, Y.; Qin, X.; Zhang, Y.; Li, D.; Ma, H.; You, H.; Li, J. Fabrication of a hollow mesoporous silica hybrid to improve the targeting of a pesticide. Chem. Eng. J. 2019, 364, 361–369. [Google Scholar] [CrossRef]
- Liu, J.-X.; Ren, J.-H.; Zhao, Q.; Shi, T.-J.; Liu, Z.-F.; Luo, Z.; Zhang, X.-G. Preparation and Characterization of Chlorpyrifos/Cyclodextrin Complex Intercalation into ZnAl-Layered Double Hydroxide. Acta Phys.-Chim. Sin. 2016, 32, 558–564. [Google Scholar] [CrossRef]
- He, F.; Zhou, Q.; Wang, L.; Yu, G.; Li, J.; Feng, Y. Fabrication of a sustained release delivery system for pesticides using interpenetrating polyacrylamide/alginate/montmorillonite nanocomposite hydrogels. Appl. Clay Sci. 2019, 183, 105347. [Google Scholar] [CrossRef]
- Yan, S.; Gu, N.; Peng, M.; Jiang, Q.; Liu, E.; Li, Z.; Yin, M.; Shen, J.; Du, X.; Dong, M. A Preparation Method of Nano-Pesticide Improves the Selective Toxicity toward Natural Enemies. Nanomaterials 2022, 12, 2419. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, W.Y.; Han, Z.H.; Wang, D.; Yin, M.Z.; Du, X.G.; Shen, J. Nanometerization of thiamethoxam by a cationic star polymer nanocarrier efficiently enhances the contact and plant-uptake dependent stomach toxicity against green peach aphids. Pest Manage. Sci. 2021, 77, 1954–1962. [Google Scholar] [CrossRef]
- Shan, Y.; Cao, L.; Muhammad, B.; Xu, B.; Zhao, P.; Cao, C.; Huang, Q. Iron-based porous metal-organic frameworks with crop nutritional function as carriers for controlled fungicide release. J. Colloid Interface Sci. 2020, 566, 383–393. [Google Scholar] [CrossRef]
- Meng, W.; Tian, Z.; Yao, P.; Fang, X.; Wu, T.; Cheng, J.; Zou, A. Preparation of a novel sustained-release system for pyrethroids by using metal-organic frameworks (MOFs) nanoparticle. Colloids Surf. A 2020, 604, 125266. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Qin, X.; Guo, Z.; Li, D.; Li, C.; Wan, H.; Zhu, F.; Li, J.; Zhang, Z.; et al. Dual stimuli-responsive fungicide carrier based on hollow mesoporous silica/hydroxypropyl cellulose hybrid nanoparticles. J. Hazard. Mater. 2021, 414, 125513. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Guo, M.; Liang, Y.; Dong, H.; Ding, G.; Zhang, W.; Tang, G.; Yang, J.; Kong, D.; Cao, Y. Pectin-conjugated silica microcapsules as dual-responsive carriers for increasing the stability and antimicrobial efficacy of kasugamycin. Carbohydr. Polym. 2017, 172, 322–331. [Google Scholar] [CrossRef]
- Xiao, D.; Liang, W.; Xie, Z.; Cheng, J.; Du, Y.; Zhao, J. A temperature-responsive release cellulose-based microcapsule loaded with chlorpyrifos for sustainable pest control. J. Hazard. Mater. 2021, 403, 123654. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Xie, Z.; Cheng, J.; Xiao, D.; Xiong, Q.; Wang, Q.; Zhao, J.; Gui, W. A Light-Triggered pH-Responsive Metal-Organic Framework for Smart Delivery of Fungicide to Control Sclerotinia Diseases of Oilseed Rape. ACS Nano 2021, 15, 6987–6997. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Gao, Y.; Wang, W.; Dong, H.; Tang, R.; Yang, J.; Niu, J.; Zhou, Z.; Jiang, N.; Cao, Y. Fabrication of smart stimuli-responsive mesoporous organosilica nano-vehicles for targeted pesticide delivery. J. Hazard. Mater. 2020, 389, 122075. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, T.M.; Qin, X.; Li, D.; Senosy, I.A.; Mmby, M.; Wan, H.; Li, J.; He, S. Pectinase-responsive carriers based on mesoporous silica nanoparticles for improving the translocation and fungicidal activity of prochloraz in rice plants. Chem. Eng. J. 2021, 404, 126440. [Google Scholar] [CrossRef]
- Ye, Z.; Guo, J.; Wu, D.; Tan, M.; Xiong, X.; Yin, Y.; He, G. Photo-responsive shell cross-linked micelles based on carboxymethyl chitosan and their application in controlled release of pesticide. Carbohydr. Polym. 2015, 132, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kaziem, A.E.; Lin, Y.; Li, C.; Tan, Y.; Huang, S.; Cheng, D.; Xu, H.; Zhang, Z. Carboxylated beta-cyclodextrin anchored hollow mesoporous silica enhances insecticidal activity and reduces the toxicity of indoxacarb. Carbohydr. Polym. 2021, 266, 118150. [Google Scholar] [CrossRef] [PubMed]
- Kaziem, A.E.; Gao, Y.; He, S.; Li, J. Synthesis and Insecticidal Activity of Enzyme-Triggered Functionalized Hollow Mesoporous Silica for Controlled Release. J. Agric. Food Chem. 2017, 65, 7854–7864. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Y.; Mao, K.; Qin, X.; Zhang, Y.; Li, D.; Zhang, Y.; Li, J.; Wan, H.; He, S. Thermoresponsive polymer-encapsulated hollow mesoporous silica nanoparticles and their application in insecticide delivery. Chem. Eng. J. 2020, 383, 123169. [Google Scholar] [CrossRef]
- Kaziem, A.E.; Gao, Y.; Zhang, Y.; Qin, X.; Xiao, Y.; Zhang, Y.; You, H.; Li, J.; He, S. alpha-Amylase triggered carriers based on cyclodextrin anchored hollow mesoporous silica for enhancing insecticidal activity of avermectin against Plutella xylostella. J. Hazard. Mater. 2018, 359, 213–221. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Wu, Y.; Wei, T.; Lu, K.; Li, L.; Lin, Y.; Wu, Y.; Huang, C.; Zhang, Y.; et al. Universal Antifouling and Photothermal Antibacterial Surfaces Based on Multifunctional Metal-Phenolic Networks for Prevention of Biofilm Formation. ACS Appl. Mater. Interfaces 2021, 13, 48403–48413. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Yang, B.J.; Jeong, K.B.; Kim, C.B.; Lee, S.; Ku, B.C. Signal-Induced Release of Guests from a Photolatent Metal-Phenolic Supramolecular Cage and Its Hybrid Assemblies. Angew.Chem. Int. Ed. Engl. 2017, 56, 5485–5489. [Google Scholar] [CrossRef]
- Liang, Y.; Song, J.; Dong, H.; Huo, Z.; Gao, Y.; Zhou, Z.; Tian, Y.; Li, Y.; Cao, Y. Fabrication of pH-responsive nanoparticles for high efficiency pyraclostrobin delivery and reducing environmental impact. Sci. Total Environ. 2021, 787, 147422. [Google Scholar] [CrossRef] [PubMed]
- Huhtamaki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, W.; Zhang, L. Seamless and automated rapeseed mapping for large cloudy regions using time-series optical satellite imagery. ISPRS J. Photogramm. 2022, 184, 45–62. [Google Scholar] [CrossRef]
- Sun, F.; Fan, G.; Hu, Q.; Zhou, Y.; Guan, M.; Tong, C.; Li, J.; Du, D.; Qi, C.; Jiang, L.; et al. The high-quality genome of Brassica napus cultivar ‘ZS11’ reveals the introgression history in semi-winter morphotype. Plant J. 2017, 92, 452–468. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Gao, Y.; Zhou, Z.; Tang, J.; Tang, G.; Niu, J.; Chen, X.; Tian, Y.; Li, Y.; Cao, Y. A simple and green preparation process for PRO@PIL-PHS-PEC microcapsules by using phosphonium ionic liquid as a multifunctional additive. Chem. Eng. J. 2021, 424, 130371. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Y.; Dong, H.; Niu, J.; Tang, J.; Yang, J.; Tang, G.; Zhou, Z.; Tang, R.; Shi, X.; et al. A Bioresponsive System Based on Mesoporous Organosilica Nanoparticles for Smart Delivery of Fungicide in Response to Pathogen Presence. ACS Sustain. Chem. Eng. 2020, 8, 5716–5723. [Google Scholar] [CrossRef]
- Xiao, D.; Cheng, J.; Liang, W.; Sun, L.; Zhao, J. Metal-phenolic coated and prochloraz-loaded calcium carbonate carriers with pH responsiveness for environmentally-safe fungicide delivery. Chem. Eng. J. 2021, 418, 129274. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Q.; Zhang, Y.; Zhu, F. Baseline Sensitivity and Toxic Actions of Prochloraz to Sclerotinia sclerotiorum. Plant Dis. 2018, 102, 2149–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, D.; Wang, Y.; Qian, Y.; Chen, C.; Jiao, B.; Cai, L.; Wang, Q. Joint acute and endocrine disruptive toxicities of malathion, cypermethrin and prochloraz to embryo-larval zebrafish, Danio rerio. Chemosphere 2017, 166, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, L.; Zhao, P.; Zhou, Z.; Cao, C.; Li, F.; Huang, Q. Emulsion-based synchronous pesticide encapsulation and surface modification of mesoporous silica nanoparticles with carboxymethyl chitosan for controlled azoxystrobin release. Chem. Eng. J. 2018, 348, 244–254. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, Y.; Tang, G.; Tian, Y.; Li, Y.; Wang, H.; Li, X.; Yu, X.; Zhang, Z.; Li, Y.; et al. Facile preparation of pH/pectinase responsive microcapsules based on CaCO3 using fungicidal ionic liquid as a nucleating agent for sustainable plant disease management. Chem. Eng. J. 2022, 446, 137073. [Google Scholar] [CrossRef]
- Xu, C.; Shan, Y.; Bilal, M.; Xu, B.; Cao, L.; Huang, Q. Copper ions chelated mesoporous silica nanoparticles via dopamine chemistry for controlled pesticide release regulated by coordination bonding. Chem. Eng. J. 2020, 395, 125093. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, Y.; Chen, X.; Li, Y.; Tian, Y.; Wang, H.; Li, X.; Yu, X.; Cao, Y. One-Pot Facile Synthesis of Double-Shelled Mesoporous Silica Microcapsules with an Improved Soft-Template Method for Sustainable Pest Management. ACS Appl. Mater. Interfaces 2021, 13, 39066–39075. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Z.; Chen, W.; Sun, L.; Yang, J.; He, K.; Dong, S.; Yuan, S. Facile pathway to construct mesoporous silica nanoparticles loaded with pyraclostrobin: Physicochemical properties, antifungal activity, and biosafety. Pest Manage. Sci. 2022, 78, 2332–2341. [Google Scholar] [CrossRef]
- Feng, J.; Sun, L.; Chen, W.; Wei, N.; Hou, C.; Chen, Z.; Meng, F.; Cao, H. Synthesis, antifungal evaluation, and safety assessment of mesoporous silica nanoparticles loaded with prothioconazole against crop pathogens. Environ. Sci. Nano 2022, 9, 2548–2558. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Yu, M.; Sun, C.; Xue, Y.; Liu, C.; Qiu, D.; Cui, B.; Zhang, Y.; Cui, H.; Zeng, Z. Tannic acid-based nanopesticides coating with highly improved foliage adhesion to enhance foliar retention. RSC Adv. 2019, 9, 27096–27104. [Google Scholar] [CrossRef] [Green Version]
- Bolton, M.D.; Thomma, B.P.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, T.; Wang, J.; Zhang, S.; Zhu, L.; Du, Z.; Wang, J. Acute and chronic toxic effects of fluoxastrobin on zebrafish (Danio rerio). Sci. Total Environ. 2018, 610, 769–775. [Google Scholar] [CrossRef] [PubMed]









| Experiment Material | Size a (nm) | BET Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|---|
| HMS | 261.4 ± 16.4 | 1187.10 | 0.36 | 3.13 |
| HMS-NH2 | 266.3 ± 16.9 | 454.01 | 0.22 | 2.96 |
| HMS-TA-Cu | 273.0 ± 15.5 | 105.70 | 0.20 | - |
| Experiment Material | Lethal Dose Probability | R2 | EC50 (μg·mL−1) | 95% Confidence Limit |
|---|---|---|---|---|
| Prochloraz Technical | Y = 1.8944x + 6.6721 | 0.9917 | 0.1411 | 0.1120–0.1532 |
| Pro@HMS-TA-Cu | Y = 1.9172x + 6.6307 | 0.9898 | 0.1310 | 0.1191–0.1671 |
| Experiment Material | Time (h) | LC50 (μg·mL−1) | 95% Confidence Limit |
|---|---|---|---|
| Prochloraz technical | 24 | 2.672 | 2.264–3.153 |
| 48 | 2.579 | 2.303–2.888 | |
| 72 | 2.205 | 2.052–2.369 | |
| 96 | 1.999 | 1.848–2.162 | |
| Pro@HMS-TA-Cu | 24 | 20.402 | 18.844–22.090 |
| 48 | 19.192 | 17.564–20.972 | |
| 72 | 17.372 | 14.087–21.422 | |
| 96 | 15.012 | 12.566–17.934 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Liang, Q.; Zang, Q.; Lv, Z.; Meng, X.; Feng, J. Construction of Prochloraz-Loaded Hollow Mesoporous Silica Nanoparticles Coated with Metal–Phenolic Networks for Precise Release and Improved Biosafety of Pesticides. Nanomaterials 2022, 12, 2885. https://doi.org/10.3390/nano12162885
Shi L, Liang Q, Zang Q, Lv Z, Meng X, Feng J. Construction of Prochloraz-Loaded Hollow Mesoporous Silica Nanoparticles Coated with Metal–Phenolic Networks for Precise Release and Improved Biosafety of Pesticides. Nanomaterials. 2022; 12(16):2885. https://doi.org/10.3390/nano12162885
Chicago/Turabian StyleShi, Liyin, Qianwei Liang, Qikai Zang, Ze Lv, Xiaohan Meng, and Jianguo Feng. 2022. "Construction of Prochloraz-Loaded Hollow Mesoporous Silica Nanoparticles Coated with Metal–Phenolic Networks for Precise Release and Improved Biosafety of Pesticides" Nanomaterials 12, no. 16: 2885. https://doi.org/10.3390/nano12162885