Effect of Silicate Additive on Structural and Electrical Properties of Germanium Nanowires Formed by Electrochemical Reduction from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
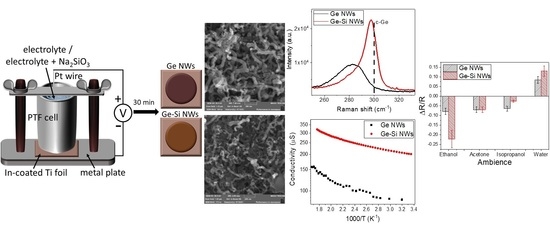
3.1. Scanning Electron Microscopy
3.2. Transmission Electron Microscopy
3.3. X-ray Photoelectron Spectroscopy
3.4. X-ray Diffraction Analysis
3.5. Raman Spectroscopy
3.6. Electrical Properties of Ge NWs Redeposited on Chips
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claeys, C.; Simoen, E. Germanium-Based Technologies from Materials to Devices, 1st ed.; Elsevier: New York, NY, USA, 2007; 480p. [Google Scholar]
- Timerkaev, B.A.; Kaleeva, A.A.; Timerkaeva, D.B.; Saifutdinov, A.I. Synthesizing Germanium Nanotubes in an Electric Arc Plasma. Russ. J. Phys. Chem. A 2020, 94, 613–617. [Google Scholar] [CrossRef]
- Wu, S.; Han, C.; Iocozzia, J.; Lu, M.; Ge, R.; Xu, R.; Lin, Z. Germanium-Based Nanomaterials for Rechargeable Batteries. Angew. Chem. Int. Ed. 2016, 55, 7898. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, T.; Brandon, M.; Ryan, K.M. Advances in the Application of Silicon and Germanium Nanowires for High-Performance Lithium-Ion Batteries. Adv. Mater. 2016, 28, 5696. [Google Scholar] [CrossRef] [PubMed]
- Mullane, E.; Kennedy, T.; Geaney, H.; Ryan, K.M. A Rapid, Solvent-Free Protocol for the Synthesis of Germanium Nanowire Lithium-Ion Anodes with a Long Cycle Life and High Rate Capability. ACS Appl. Mater. Interfaces 2014, 6, 18800. [Google Scholar] [CrossRef]
- Seo, M.H.; Park, M.; Lee, K.T.; Kim, K.; Kim, J.; Cho, J. High performance Ge nanowire anode sheathed with carbon for lithium rechargeable batteries. Energy Environ. Sci. 2011, 4, 425. [Google Scholar] [CrossRef] [Green Version]
- Kulova, T.L.; Gavrilin, I.M.; Kudryashova, Y.O.; Skundin, A.M. A LiNi0.8Co0.15Al0.05O2/Ge electrochemical system for lithium-ion batteries. Mendeleev Commun. 2020, 30, 775–776. [Google Scholar] [CrossRef]
- Gavrilin, I.M.; Kudryashova, Y.O.; Kuz’mina, A.A.; Kulova, T.L.; Skundin, A.M.; Emets, V.V.; Volkov, R.L.; Dronov, A.A.; Borgardt, N.I.; Gavrilov, S.A. High-rate and low-temperature performance of germanium nanowires anode for lithium-ion batteries. J. Electroanal. Chem. 2021, 888, 115209. [Google Scholar] [CrossRef]
- Gavrilin, I.M.; Smolyaninov, V.A.; Dronov, A.A.; Gavrilov, S.A.; Trifonov, A.Y.; Kulova, T.L.; Kuz’mina, A.A.; Skundin, A.M. Electrochemical insertion of sodium into nanostructured materials based on germanium. Mendeleev Commun. 2018, 28, 659–660. [Google Scholar] [CrossRef]
- Kim, J.; Bahk, J.-H.; Hwang, J.; Kim, H.; Park, H.; Kim, W. Thermoelectricity in semiconductor nanowires. Phys. Status Solidi RRL 2013, 7, 767. [Google Scholar] [CrossRef]
- Huang, W.; Koong, C.S.; Liang, G. Theoretical Study on Thermoelectric Properties of Ge Nanowires Based on Electronic Band Structures. IEEE Electron Device Lett. 2010, 31, 1026. [Google Scholar] [CrossRef]
- Martin, P.N.; Aksamija, Z.; Pop, E.; Ravaioli, U. Reduced Thermal Conductivity in Nanoengineered Rough Ge and GaAs Nanowires. Nano Lett. 2010, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Lee, H.S.; Cho, Y.J.; Kang, K.; Jo, M.H. Diameter-Dependent Internal Gain in Ohmic Ge Nanowire Photodetectors. Nano Lett. 2010, 10, 2043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, S.; Yang, Y.; Gould, M.; Ophir, N.; Lim, A.E.-J.; Lo, G.-Q.; Magill, P.; Bergman, K.; Baehr-Jones, T.; et al. A high-responsivity photodetector absent metal-germanium direct contact. Opt. Express 2014, 22, 11367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lee, S. Ge-Photodetectors for Si-Based Optoelectronic Integration. Sensors 2011, 11, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaisakul, P.; Marris-Morini, D.; Frigerio, J.; Chrastina, D.; Rouifed, M.S.; Cecchi, S.; Vivien, L. Integrated germanium optical interconnects on silicon substrates. Nat. Photonics 2014, 8, 482. [Google Scholar] [CrossRef]
- Yan, C.; Singh, N.; Cai, H.; Gan, C.L.; Lee, P.S. Network-Enhanced Photoresponse Time of Ge Nanowire Photodetectors. ACS Appl. Mater. Interfaces 2010, 2, 1794. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89. [Google Scholar] [CrossRef]
- O’Regan, C.; Biswas, S.; Petkov, N.; Holmes, J.D. Recent advances in the growth of germanium nanowires: Synthesis, growth dynamics and morphology control. J. Mater. Chem. C 2014, 2, 4597. [Google Scholar] [CrossRef] [Green Version]
- Collins, G.; Kolesnik, M.; Krstic, V.; Holmes, J.D. Germanium Nanowire Synthesis from Fluorothiolate-Capped Gold Nanoparticles in Supercritical Carbon Dioxide. Chem. Mater. 2010, 22, 5235. [Google Scholar] [CrossRef]
- Adhikari, H.; Marshall, A.F.; Goldthorpe, I.A.; Chidsey, C.E.; McIntyre, P.C. Metastability of Au−Ge Liquid Nanocatalysts: Ge Vapor–Liquid–Solid Nanowire Growth Far below the Bulk Eutectic Temperature. ACS Nano 2007, 1, 415. [Google Scholar] [CrossRef]
- Biswas, S.; O’Regan, C.; Petkov, N.; Morris, M.A.; Holmes, J.D. Manipulating the Growth Kinetics of Vapor–Liquid–Solid Propagated Ge Nanowires. Nano Lett. 2013, 13, 4044. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mizuta, H.; Oda, S. Growth and characterisation of Ge Nanowires by chemical vapour deposition. In Nanowires—Implementations and Applications; Hashim, A., Ed.; InTech: Rijeka, Croatia, 2011; p. 487. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, J.H.; Ratchford, J.B.; Goldthorpe, I.A.; McIntyre, P.C.; Chidsey, E.D. Vertically Oriented Germanium Nanowires Grown from Gold Colloids on Silicon Substrates and Subsequent Gold Removal. Nano Lett. 2007, 7, 1637. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Sastre, Y.; Choi, S.; Picraux, S.T.; Batt, C.A. Vertical Growth of Ge Nanowires from Biotemplated Au Nanoparticle Catalysts. J. Am. Chem. Soc. 2008, 130, 10488. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.Y.; Mata, M.; Arbiol, J.; Filler, M.A. Low-Temperature Growth of Axial Si/Ge Nanowire Heterostructures Enabled by Trisilane. Chem. Mater. 2017, 29, 3397–3402. [Google Scholar] [CrossRef] [Green Version]
- Carim, A.I.; Collins, S.M.; Foley, J.M.; Maldonado, S. Benchtop Electrochemical Liquid–Liquid–Solid Growth of Nanostructured Crystalline Germanium. J. Am. Chem. Soc. 2011, 133, 13292. [Google Scholar] [CrossRef]
- Gu, J.; Collins, S.M.; Carim, A.I.; Hao, X.; Bartlett, B.M.; Maldonado, S. Template-Free Preparation of Crystalline Ge Nanowire Film Electrodes via an Electrochemical Liquid–Liquid–Solid Process in Water at Ambient Pressure and Temperature for Energy Storage. Nano Lett. 2012, 12, 4617. [Google Scholar] [CrossRef]
- Ma, L.; Gu, J.; Fahrenkrug, E.; Maldonado, S.J. Electrochemical Liquid-Liquid-Solid Deposition of Crystalline Ge Nanowires as a Function of Ga Nanodroplet Size. Electrochem. Soc. 2014, 161, D3044. [Google Scholar] [CrossRef]
- Mahenderkar, N.K.; Liu, Y.-C.; Koza, J.A.; Switzer, J.A. Electrodeposited Germanium Nanowires. ACS Nano 2014, 8, 9524. [Google Scholar] [CrossRef]
- Gavrilov, S.A.; Dronov, A.A.; Gavrilin, I.M.; Volkov, R.L.; Borgardt, N.I.; Trifonov, A.Y.; Pavlikov, A.V.; Forsh, P.A.; Kashkarov, P.K. Laser crystallization of germanium nanowires fabricated by electrochemical deposition. J. Raman Spectrosc. 2018, 49, 810–816. [Google Scholar] [CrossRef]















| Sample | C | O | Ge | In | K | Na | Si | In/Ge | O/Ge | K/Ge | Na/Ge | Si/Ge |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ge NWs | 26.8 | 33.6 | 29.7 | 2.4 | 0.9 | 3.6 | 0 | 0.08 | 1.1 | 0.03 | 0.12 | 0 |
| Ge-Si NWs | 40.0 | 32.7 | 20.0 | 1.5 | 2.1 | 2.1 | 1.6 | 0.08 | 1.6 | 0.11 | 0.11 | 0.08 |
| Ge-Si NWs after 3 min sputtering | 0 | 26.0 | 54.7 | 2.3 | 4.2 | 3.7 | 1.2 | 0.04 | 0.5 | 0.08 | 0.07 | 0.02 |
| Surface State | Ge3d | In3d5 | |||||
|---|---|---|---|---|---|---|---|
| 1 Ge0 | 2 GeO | 3 GeO2 | 1 In0 | 2 In-Ge | 3 In2O3/In(OH)3 | ||
| Initial | Eb, eV | 29.4 | 30.5 | 33.0 | 443.9 | - | 445.4 |
| Relative peak intensity | 53 | 23 | 24 | 40 | - | 60 | |
| After 3 min sputtering | Eb, eV | 29.4 | 30.5 | 32.5 | - | 444.2 | - |
| Relative peak intensity | 75 | 20 | 5 | - | 100 | - | |
| Sample, Conditions | a × 105 | b × 108 | m |
|---|---|---|---|
| Ge NWs, vacuum | 6.4 | 3.4 | 2.52 |
| Ge NWs, air | 5.2 | 3.4 | 2.32 |
| Ge NWs, air, 39 days | 2.0 | 9.7 | 2.12 |
| Ge-Si NWs, vacuum | 22 | 2200 | 1.94 |
| Ge-Si NWs, air | 11.4 | - | - |
| Ge-Si NWs, air, 19 days | 4.6 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eremina, A.S.; Gavrilin, I.M.; Pokryshkin, N.S.; Kharin, A.Y.; Syuy, A.V.; Volkov, V.S.; Yakunin, V.G.; Bubenov, S.S.; Dorofeev, S.G.; Gavrilov, S.A.; et al. Effect of Silicate Additive on Structural and Electrical Properties of Germanium Nanowires Formed by Electrochemical Reduction from Aqueous Solutions. Nanomaterials 2022, 12, 2884. https://doi.org/10.3390/nano12162884
Eremina AS, Gavrilin IM, Pokryshkin NS, Kharin AY, Syuy AV, Volkov VS, Yakunin VG, Bubenov SS, Dorofeev SG, Gavrilov SA, et al. Effect of Silicate Additive on Structural and Electrical Properties of Germanium Nanowires Formed by Electrochemical Reduction from Aqueous Solutions. Nanomaterials. 2022; 12(16):2884. https://doi.org/10.3390/nano12162884
Chicago/Turabian StyleEremina, Anna S., Ilya M. Gavrilin, Nikolay S. Pokryshkin, Alexander Yu. Kharin, Alexander V. Syuy, Valentin S. Volkov, Valery G. Yakunin, Sergei S. Bubenov, Sergey G. Dorofeev, Sergey A. Gavrilov, and et al. 2022. "Effect of Silicate Additive on Structural and Electrical Properties of Germanium Nanowires Formed by Electrochemical Reduction from Aqueous Solutions" Nanomaterials 12, no. 16: 2884. https://doi.org/10.3390/nano12162884