Synthesis of Superhydrophobic Cellulose Stearoyl Ester for Oil/Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
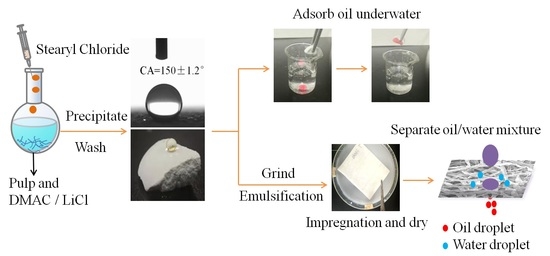
2.2. Preparation of CSE and OS/CSE Latex
2.3. Characterization
3. Results and Discussion
3.1. Preparation of CSE and OS/CSE@FBP Membrane
3.2. FTIR and EDS Analysis of CSE and OS/CSE@FBP Membrane
3.3. Wettability of CSE and OS/CSE@FBP Membrane
3.4. Surface Morphologies of CSE and OS/CSE@FBP Membranes
3.5. Oil Adsorption Analysis of CSE
3.6. Oil–Water Separation of OS/CSE@FBP Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, L.; Wang, B.; Zeng, J.; Cheng, Z.; Li, J.; Xu, J.; Gao, W.; Chen, K. Degradable dual superlyophobic lignocellulosic fibers for high-efficiency oil/water separation. Green Chem. 2020, 22, 504–512. [Google Scholar] [CrossRef]
- Li, Z.; Bo, W.; Qin, X.; Wang, Y.; Liu, C.; Qian, S.; Ning, W.; Zhang, J.; Shen, C.; Guo, Z. Superhydrophobic/Superoleophilic Polycarbonate/Carbon Nanotubes Porous Monolith for Selective Oil Adsorption from Water. ACS Sustain. Chem. Eng. 2018, 6, 13747–13755. [Google Scholar] [CrossRef]
- Cheryan, M.; Rajagopalan, N. Membrane processing of oily streams. Wastewater treatment and waste reduction. J. Membr. Sci. 2015, 151, 13–28. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Hou, X.; Chen, F. Emerging Separation Applications of Surface Superwettability. Nanomaterials 2022, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A Review on Oil/Water Mixture Separation Material. Ind. Eng. Chem. Res. 2020, 59, 14546–14568. [Google Scholar] [CrossRef]
- Song, J.; Liu, N.; Li, J.; Cao, Y.; Cao, H. Facile Fabrication of Highly Hydrophobic Onion-like Candle Soot-Coated Mesh for Durable Oil/Water Separation. Nanomaterials 2022, 12, 761. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, K.; Aubry, C.; Zou, L. Amphiphilic Janus 3D MoS2/rGO Nanocomposite for Removing Oil from Wastewater. Ind. Eng. Chem. Res. 2021, 60, 1266–1273. [Google Scholar] [CrossRef]
- Xu, L.P.; Wu, X.; Meng, J.; Peng, J.; Wen, Y.; Zhang, X.; Wang, S. Papilla-like magnetic particles with hierarchical structure for oil removal from water. Chem. Commun. 2013, 49, 8752–8754. [Google Scholar] [CrossRef]
- Sarkar, A.; Mahapatra, S. Novel hydrophobic vaterite particles for oil removal and recovery. J. Mater. Chem. A 2014, 2, 3808–3818. [Google Scholar] [CrossRef]
- Yu, L.; Hao, G.; Liang, Q.; Zhou, S.; Zhang, N.; Jiang, W. Facile preparation and characterization of modified magnetic silica nanocomposite particles for oil absorption. Appl. Surf. Sci. 2015, 357, 2297–2305. [Google Scholar] [CrossRef]
- Arbatan, T.; Fang, X.; Shen, W. Superhydrophobic and oleophilic calcium carbonate powder as a selective oil sorbent with potential use in oil spill clean-ups. Chem. Eng. J. 2011, 166, 787–791. [Google Scholar] [CrossRef]
- Eduok, U. New superhydrophobic and self-cleaning zirconia/polydimethylsiloxane nanocomposite coated cotton fabrics. New J. Chem. 2021, 45, 638–650. [Google Scholar] [CrossRef]
- Yao, H.; Lu, X.; Chen, S.; Yu, C.; Xin, Z. A Robust Polybenzoxazine/SiO2 Fabric with Superhydrophobicity for High-Flux Oil/Water Separation. Ind. Eng. Chem. Res. 2020, 59, 7787–7796. [Google Scholar] [CrossRef]
- He, J.; Zhao, H.; Li, X.; Su, D.; Zhang, F.; Ji, H.; Liu, R. Superelastic and superhydrophobic bacterial cellulose/silica aerogels with hierarchical cellular structure for oil absorption and recovery. J. Hazard. Mater. 2018, 346, 199–207. [Google Scholar] [CrossRef]
- Thangavelu, K.; Ravaux, F.; Zou, L. Cellulose acetate-MoS2 amphiphilic Janus-like fibrous sponge for removing oil from wastewater. Environ. Technol. Innov. 2021, 24, 101870. [Google Scholar] [CrossRef]
- Thangavelu, K.; Zou, L. Evaluating oil removal by amphiphilic MoS2/cellulose acetate fibrous sponge in a flow-through reactor and by artificial neural network. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100684. [Google Scholar] [CrossRef]
- Bagoole, O.; Rahman, M.M.; Shah, S.; Hong, H.; Chen, H.; Al Ghaferi, A.; Younes, H. Functionalized three-dimensional graphene sponges for highly efficient crude and diesel oil adsorption. Environ. Sci. Pollut. Res. Int. 2018, 25, 23091–23105. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhao, X.; Weng, Y.; Li, Y.; Zeng, J. Fully Sustainable, Nanoparticle-Free, Fluorine-Free, and Robust Superhydrophobic Cotton Fabric Fabricated via an Eco-Friendly Method for Efficient Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 15696–15705. [Google Scholar] [CrossRef]
- Satgé, C.; Granet, R.; Verneuil, B.; Branland, P.; Krausz, P. Synthesis and properties of biodegradable plastic films obtained by microwave-assisted cellulose acylation in homogeneous phase. Comptes Rendus Chim. 2004, 7, 135–142. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, X.; White, K.L.; Lin, S.; Wu, H.; Cao, S.; Huang, L.; Chen, L. Effect of the degree of substitution on the hydrophobicity of acetylated cellulose for production of liquid marbles. Cellulose 2016, 23, 811–821. [Google Scholar] [CrossRef]
- Willberg-Keyriläinen, P.; Vartiainen, J.; Harlin, A.; Ropponen, J. The effect of side-chain length of cellulose fatty acid esters on their thermal, barrier and mechanical properties. Cellulose 2016, 24, 505–517. [Google Scholar] [CrossRef]
- Geissler, A.; Loyal, F.; Biesalski, M.; Zhang, K. Thermo-responsive superhydrophobic paper using nanostructured cellulose stearoyl ester. Cellulose 2014, 21, 357–366. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Wang, W.; Zeng, L.; Wang, S.; Qin, C.; Qin, C. Modulation of superhydrophobicity and self-binding strength of cellulose ester-based coating by changing the degree of substitution. J. Mater. Sci. 2021, 56, 5924–5935. [Google Scholar] [CrossRef]
- Hubbe, M. Bonding between cellulosic fibers in the absence and presence of dry-strength agents—A review. BioResources 2006, 1, 281–318. [Google Scholar] [CrossRef]
- Liebert, T.F.; Heinze, T.J. Exploitation of Reactivity and Selectivity in Cellulose Functionalization Using Unconventional Media for the Design of Products Showing New Superstructures. Biomacromolecules 2001, 2, 1124–1132. [Google Scholar] [CrossRef]
- Grote, C.; Heinze, T. Starch Derivatives of High Degree of Functionalization 11: Studies on Alternative Acylation of Starch with Long-chain Fatty Acids Homogeneously in N,N-dimethyl acetamide/LiCl. Cellulose 2005, 12, 435–444. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Nishiyama, Y.; Kuga, S. Surface acetylation of bacterial cellulose. Cellulose 2002, 9, 361–367. [Google Scholar] [CrossRef]
- Li, D.; Li, Q.; Mao, D.; Bai, N.; Dong, H. A versatile bio-based material for efficiently removing toxic dyes, heavy metal ions and emulsified oil droplets from water simultaneously. Bioresour. Technol. 2017, 245, 649–655. [Google Scholar] [CrossRef]
- Churipard, S.R.; Kanakikodi, K.S.; Rambhia, D.A.; Raju, C.S.K.; Halgeri, A.B.; Choudary, N.V.; Ganesh, G.S.; Ravishankar, R.; Maradur, S.P. Porous polydivinylbenzene (PDVB) as an efficient adsorbent for hydrocarbons: Effect of porogens on adsorption capacity. Chem. Eng. J. 2020, 380, 122481. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Wang, W.; Wang, S.; Qin, C. Reactive superhydrophobic paper from one-step spray-coating of cellulose-based derivative. Appl. Surf. Sci. 2019, 497, 143816. [Google Scholar] [CrossRef]
- Xu, C.L.; Wang, Y.Z. One-Step Approach to the Growth of ZnO Nano-/Microrods on Cellulose toward Its Durable Superhydrophobicity. Adv. Mater. Interfaces 2017, 4, 1700550. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Samyn, P. Synthesis of Polyhydroxybutyrate Particles with Micro-to-Nanosized Structures and Application as Protective Coating for Packaging Papers. Nanomaterials 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Jo, W.H.; Kwon, I.C.; Kim, Y.-H.; Jeong, S.Y. Physicochemical characteristics of self-aggregates of hydrophobically modified chitosans. Langmuir 1998, 14, 2329–2332. [Google Scholar] [CrossRef]
- Eungjin, A.; Taehyung, K.; Yuju, J.; Byeong-Su, K. A4 Paper Chemistry: Synthesis of a Versatile and Chemically Modifiable Cellulose Membrane. ACS Nano 2020, 14, 6173–6180. [Google Scholar]






| Unit | Filter Base Paper | OS/CSE@FBP Membrane | |
|---|---|---|---|
| Maximum pore size | µm | 38.1 | 25.3 |
| Mean pore size | µm | 13.7 | 10.4 |
| Minimum pore size | µm | 4.6 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Su, W.; Hu, J.; Xu, Y.; Liu, Z.; Hui, L. Synthesis of Superhydrophobic Cellulose Stearoyl Ester for Oil/Water Separation. Nanomaterials 2022, 12, 1964. https://doi.org/10.3390/nano12121964
Yang Q, Su W, Hu J, Xu Y, Liu Z, Hui L. Synthesis of Superhydrophobic Cellulose Stearoyl Ester for Oil/Water Separation. Nanomaterials. 2022; 12(12):1964. https://doi.org/10.3390/nano12121964
Chicago/Turabian StyleYang, Qian, Weiyin Su, Jianquan Hu, Yan Xu, Zhong Liu, and Lanfeng Hui. 2022. "Synthesis of Superhydrophobic Cellulose Stearoyl Ester for Oil/Water Separation" Nanomaterials 12, no. 12: 1964. https://doi.org/10.3390/nano12121964