Ultralight Open-Cell Graphene Aerogels with Multiple, Gradient Microstructures for Efficient Microwave Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Controllable Preparation of OCGA
2.3. Preparation of OCGA-G
2.4. Characterization
2.5. Electromagnetic Parameters Measurement and Microwave Absorption Calculation
3. Results and Discussion
3.1. Fabrication of OCGAs
3.2. Testing and Characterization
3.3. Effect of Graphene Framework on the Microwave Absorption Performance
3.4. Effect of Open-Cell Structure on the Microwave Absorption Performance
3.4.1. Effect of Cell Number on Microwave Absorption Performance
3.4.2. Effect of Cell Size on Microwave Absorption Performance
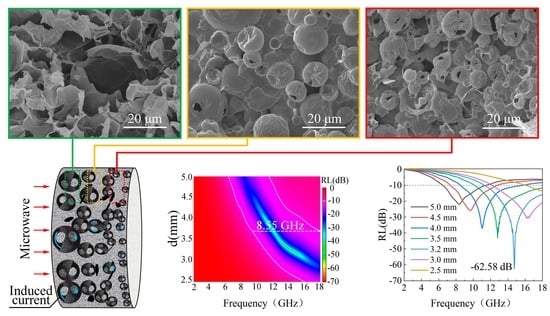
3.5. OCGA with Gradient Structure for Efficient Microwave Absorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhi, D.; Li, T.; Li, J.; Ren, H.; Meng, F. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Part B Eng. 2021, 211, 108642. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zong, Y.; Tan, G.; Sun, Y.; Lan, Y.; He, M.; Ren, Z.; Zheng, X. Solvothermal synthesis of nitrogen-doped graphene decorated by superparamagnetic Fe3O4 nanoparticles and their applications as enhanced synergistic microwave absorbers. Carbon 2017, 115, 493–502. [Google Scholar] [CrossRef]
- Yao, D.; Peng, N.; Zheng, Y. Enhanced mechanical and thermal performances of epoxy resin by oriented solvent-free graphene/carbon nanotube/Fe3O4 composite nanofluid. Compos. Sci. Technol. 2018, 167, 234–242. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Xu, P.; Zhang, Y.; Yue, X. Porous carbon/graphite nanosheet/ferromagnetic nanoparticle composite absorbents with adjustable electromagnetic properties. Nanotechnology 2021, 32, 205707. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Huang, M.; You, W.; Zeng, Q.; Zhang, J.; Liu, X.; Wang, M.; Che, R. Orientation growth modulated magnetic-carbon microspheres toward broadband electromagnetic wave absorption. Carbon 2021, 172, 516–528. [Google Scholar] [CrossRef]
- Tang, J.; Liang, N.; Wang, L.; Li, J.; Tian, G.; Zhang, D.; Feng, S.; Yue, H. Three-dimensional nitrogen-doped reduced graphene oxide aerogel decorated with Ni nanoparticles with tunable and unique microwave absorption. Carbon 2019, 152, 575–586. [Google Scholar] [CrossRef]
- Yuan, H.; Yan, F.; Li, C.; Zhu, C.; Zhang, X.; Chen, Y. Nickel nanoparticle encapsulated in few-layer nitrogen-doped graphene supported by nitrogen-doped graphite sheets as a high-performance electromagnetic wave absorbing material. ACS Appl. Mater. Interfaces 2018, 10, 1399–1407. [Google Scholar] [CrossRef]
- Xu, D.; Yang, S.; Chen, P.; Yu, Q.; Xiong, X.; Wang, J. Synthesis of magnetic graphene aerogels for microwave absorption by in-situ pyrolysis. Carbon 2019, 146, 301–312. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Lv, X.; Cui, G.; Gu, G. Facile synthesis 3D porous MXene Ti3C2Tx@RGO composite aerogel with excellent dielectric loss and electromagnetic wave absorption. J. Alloys Compd. 2020, 828, 154251. [Google Scholar] [CrossRef]
- Liang, L.; Li, Q.; Yan, X.; Feng, Y.; Wang, Y.; Zhang, H.-B.; Zhou, X.; Liu, C.; Shen, C.; Xie, X. Multifunctional magnetic Ti3C2Tx MXene/graphene aerogel with superior electromagnetic wave absorption performance. ACS Nano 2021, 15, 6622–6632. [Google Scholar] [CrossRef]
- Chen, J.-P.; Jia, H.; Liu, Z.; Kong, Q.-Q.; Hou, Z.-H.; Xie, L.-J.; Sun, G.-H.; Zhang, S.-C.; Chen, C.-M. Construction of C-Si heterojunction interface in SiC Whisker/reduced graphene oxide aerogels for improving microwave absorption. Carbon 2020, 164, 59–68. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Fu, Y.; Wu, X.; Wang, Q.; Zhang, W.; Luo, C. Enhanced microwave absorption performances of polyaniline/graphene aerogel by covalent bonding. Compos. Part B Eng. 2019, 169, 221–228. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Wang, L.; Ren, J.; Xu, Y. Ultralight graphene aerogel enhanced with transformed micro-structure Led by Polypyrrole nano-rods and its improved microwave absorption properties. Compos. Part A Appl. Sci. Manuf. 2017, 97, 141–150. [Google Scholar] [CrossRef]
- Ma, J.; Li, W.; Fan, Y.; Yang, J.; Yang, Q.; Wang, J.; Luo, W.; Zhou, W.; Nomura, N.; Wang, L.; et al. Ultrathin and light-weight graphene aerogel with precisely tunable density for highly efficient microwave absorbing. ACS Appl. Mater. Interfaces 2019, 11, 46386–46396. [Google Scholar] [CrossRef]
- Huang, X.; Yu, G.; Zhang, Y.; Zhang, M.; Shao, G. Design of cellular structure of graphene aerogels for electromagnetic wave absorption. Chem. Eng. J. 2021, 426, 131894. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhou, Z.-H.; Zhu, J.-L.; Sun, W.-J.; Yan, D.-X.; Dai, K.; Li, Z.-M. Low-temperature carbonized carbon nanotube/cellulose aerogel for efficient microwave absorption. Compos. Part B Eng. 2021, 220, 108985. [Google Scholar] [CrossRef]
- González, M.; Baselga, J.; Pozuelo, J. Modulating the electromagnetic shielding mechanisms by thermal treatment of high porosity graphene aerogels. Carbon 2019, 147, 27–34. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, K.; Song, S.; Tsiakaras, P. 3D interconnected hierarchically porous N-doped carbon with NH3 activation for efficient oxygen reduction reaction. Appl. Catal. 2017, 210, 57–66. [Google Scholar] [CrossRef]
- Cao, M.-S.; Yang, J.; Song, W.-L.; Zhang, D.-Q.; Wen, B.; Jin, H.-B.; Hou, Z.-L.; Yuan, J. Ferroferric oxide/multiwalled carbon nanotube vs polyaniline/ferroferric oxide/multiwalled carbon nanotube multiheterostructures for highly effective microwave absorption. ACS Appl. Mater. Interfaces 2012, 4, 6949–6956. [Google Scholar] [CrossRef]
- Kim, S.; Jo, S.; Gueon, K.; Choi, K.; Kim, J.; Churn, K. Complex permeability and permittivity and microwave absorption of ferrite-rubber composite at X-band frequencies. IEEE Trans. Magn. 1991, 27, 5462–5464. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Gu, J.; Liu, H.; Liu, C.; Luo, C.; Kong, J.; Shao, Q.; Wang, N.; Guo, Z.; et al. Ultralight, highly compressible and fire-retardant graphene aerogel with self-adjustable electromagnetic wave absorption. Carbon 2018, 139, 1126–1135. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, R.; Jiao, W.; Ding, G.; Hao, L.; Yang, F.; He, X. MoS2 graphene fiber based gas sensing devices. Carbon 2015, 95, 34–41. [Google Scholar] [CrossRef]
- Ding, G.; Jiao, W.; Wang, R.; Niu, Y.; Hao, L.; Yang, F.; Liu, W. A biomimetic, multifunctional, superhydrophobic graphene film with self-sensing and fast recovery properties for microdroplet transportation. J. Mater. Chem. A 2017, 5, 17325–17334. [Google Scholar] [CrossRef]
- Kuang, B.; Ning, M.; Wang, L.; Li, J.; Wang, C.; Hou, Z.; Zhao, Y.; Jin, H. Biopolymer nanofiber/reduced graphene oxide aerogels for tunable and broadband high-performance microwave absorption. Compos. Part B Eng. 2019, 161, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Chen, X.; Wu, Z.; He, Y.; Lv, Y.; Zou, Y. Broadband metamaterial absorber for low-frequency microwave absorption in the S-band and C-band. J. Magn. Magn. Mater. 2020, 497, 166075. [Google Scholar] [CrossRef]
- Ding, G.; Jiao, W.; Wang, R.; Niu, Y.; Chen, L.; Hao, L. Ultrafast, reversible transition of superwettability of graphene network and controllable underwater oil adhesion for oil microdroplet transportation. Adv. Funct. Mater. 2018, 28, 1706686. [Google Scholar] [CrossRef]
- Ding, G.; Jiao, W.; Wang, R.; Yan, M.; Chu, Z.; He, X. Superhydrophobic heterogeneous graphene networks with controllable adhesion behavior for detecting multiple underwater motions. J. Mater. Chem. A 2019, 7, 17766–17774. [Google Scholar] [CrossRef]
- Guo, H.-L.; Wang, X.-F.; Qian, Q.-Y.; Wang, F.-B.; Xia, X.-H. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Zhang, D. Aqueous colloids of graphene oxide nanosheets by exfoliation of graphite oxide without ultrasonication. Bull. Mater. Sci. 2011, 34, 25–28. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Lin, Z. Functionalized Graphene for Energy Storage and Conversion. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2014. [Google Scholar]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–Phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Scardaci, V.; Compagnini, G. Raman spectroscopy investigation of graphene oxide reduction by laser scribing. C 2021, 7, 48. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using raman spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allahbakhsh, A.; Sharif, F.; Mazinani, S.; Kalaee, M. Synthesis and characterization of graphene oxide in suspension and powder forms by chemical exfoliation method. Int. J. Nano Dimens. 2014, 5, 11–20. [Google Scholar]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Du, Y.; Liu, W.; Qiang, R.; Wang, Y.; Han, X.; Ma, J.; Xu, P. Shell thickness-dependent microwave absorption of core–shell Fe3O4@C composites. ACS Appl. Mater. Interfaces 2014, 6, 12997–13006. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Yan, J.; Huang, Y.; Xia, L.; Guang, Z. Synthesis of lightweight N-doped graphene foams with open reticular structure for high-efficiency electromagnetic wave absorption. Chem. Eng. J. 2019, 368, 285–298. [Google Scholar] [CrossRef]
- Luo, F.; Liu, D.; Cao, T.; Cheng, H.; Kuang, J.; Deng, Y.; Xie, W. Study on broadband microwave absorbing performance of gradient porous structure. Adv. Compos. Hybrid. Mater. 2021, 4, 591–601. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, Q.; Xiao, H.; Ding, G.; Liu, H.; Zhao, C.; Wang, R.; Huang, Z. Ultralight Open-Cell Graphene Aerogels with Multiple, Gradient Microstructures for Efficient Microwave Absorption. Nanomaterials 2022, 12, 1896. https://doi.org/10.3390/nano12111896
Mei Q, Xiao H, Ding G, Liu H, Zhao C, Wang R, Huang Z. Ultralight Open-Cell Graphene Aerogels with Multiple, Gradient Microstructures for Efficient Microwave Absorption. Nanomaterials. 2022; 12(11):1896. https://doi.org/10.3390/nano12111896
Chicago/Turabian StyleMei, Qilin, Han Xiao, Guomin Ding, Huizhi Liu, Chenglong Zhao, Rui Wang, and Zhixiong Huang. 2022. "Ultralight Open-Cell Graphene Aerogels with Multiple, Gradient Microstructures for Efficient Microwave Absorption" Nanomaterials 12, no. 11: 1896. https://doi.org/10.3390/nano12111896