Study of Tribological Properties of Fullerenol and Nanodiamonds as Additives in Water-Based Lubricants for Amorphous Carbon (a-C) Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fullerenol Preparation
2.3. Tribological Evaluation
2.4. Characterizations
3. Results
3.1. Fullerenol and Nanodiamond Characterizations
3.2. Tribological Results
3.3. Characterizations of Worn a-C Surfaces
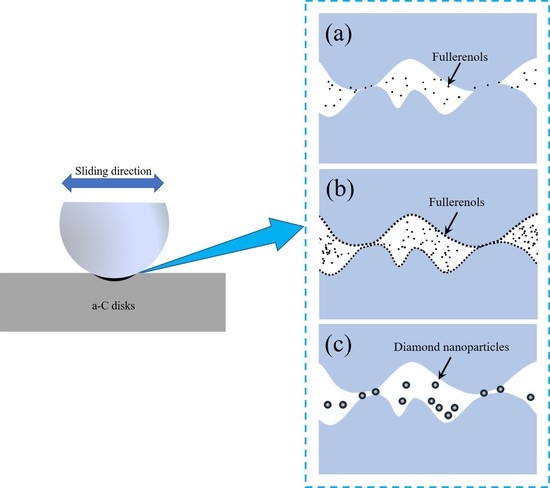
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chinas-Castillo, F.; Lara-Romero, J.; Alonso-Nunez, G.; Barceinas-Sanchez, J.D.O.; Jimenez-Sandoval, S. Friction reduction by water-soluble ammonium thiometallates. Tribol. Lett. 2007, 26, 137–144. [Google Scholar] [CrossRef]
- Lebedev, V.T.; Grushko, Y.S.; Orlova, D.N.; Kim, A.F.; Kozlov, V.S.; Sedov, V.P.; Kolesnik, S.G.; Shamanin, V.V.; Melenevskaya, E.Y. Aggregation in hydroxylated endohedral fullerene solutions. Fuller. Nanotub. Car. Nanostruct. 2010, 18, 422–426. [Google Scholar] [CrossRef]
- Nikolaev, I.V.; Lebedev, V.T.; Grushko, Y.S.; Sedov, V.P.; Shilin, V.A.; Török, G.; Melenevskaya, E.Y. Ordering of hydroxylated fullerenes in aqueous solutions. Fuller. Nanotub. Car. Nanostruct. 2012, 20, 345–350. [Google Scholar] [CrossRef]
- Wu, P.; Chen, X.; Zhang, C.; Luo, J. Synergistic tribological behaviors of graphene oxide and nanodiamond as lubricating additives in water. Tribol. Int. 2019, 132, 177–184. [Google Scholar] [CrossRef]
- Cheng, G.; Dong, L.; Kamboj, L.; Khosla, T.; Wang, X.; Zhang, Z.; Guo, L.; Pesika, N.; Ding, J. Hydrothermal synthesis of monodisperse hard carbon spheres and their water-based lubrication. Tribol. Lett. 2017, 65, 141. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, F.; Ding, X.; Zhou, Z.; Wang, C.; Zhang, W.; Li, L.K.-Y.; Lee, S.-T. Structure and water-lubricated tribological properties of Cr/a-C coatings with different Cr contents. Tribol. Int. 2013, 67, 104–115. [Google Scholar] [CrossRef]
- Li, H.; Xu, T.; Wang, C.; Chen, J.; Zhou, H.; Liu, H. Tribochemical effects on the friction and wear behaviors of a-C:H and a-C films in different environment. Tribol. Int. 2007, 40, 132–138. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, J.; Wang, L.; Xue, Q. Synergistic effect of hybrid carbon nanotube–graphene oxide as nanoadditive enhancing the frictional properties of ionic liquids in high vacuum. ACS Appl. Mater. Interfaces 2015, 7, 8592–8600. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, J.; Luo, T.; Cao, B.; Xu, J.; Chen, X.; Luo, J. Tribochemical mechanism of superlubricity in graphene quantum dots modified DLC films under high contact pressure. Carbon 2021, 173, 329–338. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, J.; Wang, L.; Xue, Q. Frictional dependence of graphene and carbon nanotube in diamond-like carbon/ionic liquids hybrid films in vacuum. Carbon 2014, 80, 734–745. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Wang, C.; Ye, Y.; Zhao, H.; Li, J.; Lu, X.; Mao, C.; Chen, S.; Mao, J.; et al. One-pot pyrolysis preparation of carbon dots as eco-friendly nanoadditives of water-based lubricants. Carbon 2019, 152, 511–520. [Google Scholar] [CrossRef]
- Elomaa, O.; Singh, V.K.; Iyer, A.; Hakala, T.J.; Koskinen, J. Graphene oxide in water lubrication on diamond-like carbon vs. stainless steel high-load contacts. Diam. Relat. Mater. 2015, 52, 43–48. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, S.; Sun, Y.; Yue, W.; Zhang, H. Bioinspired surface functionalization of nanodiamonds for enhanced lubrication. Langmuir 2018, 34, 12436–12444. [Google Scholar] [CrossRef]
- Kogovsek, J.; Kalin, M. Various MoS2−, WS2− and C-Based micro- and nanoparticles in boundary lubrication. Tribol. Lett. 2014, 53, 585–597. [Google Scholar] [CrossRef]
- Tomala, A.; Karpinska, A.; Werner, W.S.M.; Olver, A.; Störi, H. Tribological properties of additives for water-based lubricants. Wear 2010, 269, 804–810. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Tang, W.; Wang, B. Remarkable lubricating effect of ionic liquid modified carbon dots as a kind of water-based lubricant additives. Nano 2017, 12, 1750108. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S.; Xu, Q.; Zhang, H. Carbon quantum dots: An innovative additive for water lubrication. Sci. China Technol. Sc. 2019, 62, 587–596. [Google Scholar] [CrossRef]
- Tang, J.; Chen, S.; Jia, Y.; Ma, Y.; Xie, H.; Quan, X.; Ding, Q. Carbon dots as an additive for improving performance in water-based lubricants for amorphous carbon (a-C) coatings. Carbon 2020, 156, 272–281. [Google Scholar] [CrossRef]
- Wang, B.; Tang, W.; Lu, H.; Huang, Z. Ionic liquid capped carbon dots as a high-performance friction-reducing and antiwear additive for poly (ethylene glycol). J. Mater. Chem. A 2016, 4, 7257–7265. [Google Scholar] [CrossRef]
- Qiang, R.; Hu, L.; Hou, K.; Wang, J.; Yang, S. Water-soluble graphene quantum dots as high-performance water-based lubricant additive for steel/steel contact. Tribol. Lett. 2019, 67, 64. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Liu, P.; Zheng, J.; Shu, C.; Pan, G.; Luo, J. Modification on the tribological properties of ceramics lubricated by water using fullerenol as a lubricating additive. Sci. China Technol. Sci. 2012, 55, 2656–2661. [Google Scholar] [CrossRef]
- Fortner, J.D.; Lyon, D.Y.; Sayes, C.M.; Boyd, A.M.; Falkner, J.C.; Hotze, E.M.; Alemany, L.B.; Tao, Y.J.; Guo, W.; Ausman, K.D.; et al. C60 in water: Nanocrystal formation and microbial response. Environ. Sci. Technol. 2005, 39, 4307–4316. [Google Scholar] [CrossRef] [PubMed]
- Semenov, K.N.; Charykov, N.A.; Keskinov, V.N. Fullerenol synthesis and identification. properties of the fullerenol water solutions. J. Chem. Eng. Data 2011, 56, 230–239. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Lu, Z.; Gao, X. Syntheses, structures and antioxidant activities of fullerenols: Knowledge learned at the atomistic level. J. Clust. Sci. 2015, 26, 375–388. [Google Scholar] [CrossRef]
- Sharoyko, V.V.; Ageev, S.V.; Meshcheriakov, A.A.; Akentiev, A.V.; Noskov, B.A.; Rakipov, I.T.; Charykov, N.A.; Kulenova, N.A.; Shaimardanova, B.K.; Podolsky, N.E.; et al. Physicochemical study of water-soluble C60(OH)24 fullerenol. J. Mol. Liq. 2020, 311, 113360. [Google Scholar] [CrossRef]
- Bo, F. Relationship between the structure of C60 and its lubricity: A review. Lubr. Sci. 1997, 9, 181–193. [Google Scholar] [CrossRef]
- McHedlov-Petrossyan, N.O. Fullerenes in aqueous media: A review. Theor. Exp. Chem. 2020, 55, 361–391. [Google Scholar] [CrossRef]
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N.; Panova, G.G. Fullerenol can ameliorate iron deficiency in cucumber grown hydroponically. J. Plant Growth Regul. 2021, 40, 1017–1031. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Chang, C.-Y.; Liu, J.-C.; Chen, J.-H.; So, E.C.; Wu, P.-C. Two-photon photoexcited photodynamic therapy with water-soluble fullerenol serving as the highly effective two-photon photosensitizer against multidrug-resistant bacteria. Int. J. Nanomed. 2020, 15, 6813–6825. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Wang, J.-Y.; Chang, C.-Y.; Liu, J.-C.; Shao, Y.-T.; Lin, Y.-S.; So, E.C.; Wu, P.-C. Water-soluble fullerenol with hydroxyl group dependence for efficient two-photon excited photodynamic inactivation of infectious microbes. Nanoscale Res. Lett. 2020, 15, 99. [Google Scholar]
- Kokubo, K.; Shirakawa, S.; Kobayashi, N.; Aoshima, H.; Oshima, T. Facile and scalable synthesis of a highly hydroxylated water-soluble fullerenol as a single nanoparticle. Nano Res. 2011, 4, 204–215. [Google Scholar] [CrossRef]
- Stankovic, B.; Jovanovic, J.; Adnadjevic, B. Application of the suzuki–fraser function in modelling the non-isothermal dehydroxylation kinetics of fullerol. React. Kinet. Mech. Cat. 2018, 123, 421–438. [Google Scholar] [CrossRef]
- Wang, F.F.; Wang, C.; Liu, R.Q.; Tian, D.; Li, N. Experimental study on the preparation of Ag nanoparticle doped fullerenol for lithium ion battery application. J. Phys. Chem. C 2012, 116, 10461–10467. [Google Scholar] [CrossRef]
- Xu, J.; Yokota, Y.; Wong, R.A.; Kim, Y.; Einaga, Y. Unusual electrochemical properties of low-doped boron-doped diamond electrodes containing sp2 carbon. J. Am. Chem. Soc. 2020, 142, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yang, N.; Yang, B.; Schulte, A.; Jin, Q.; Koch, U.; Mandal, S.; Engelhard, C.; Williams, O.A.; Schönherr, H.; et al. Electrochemistry of nitrogen and boron Bi-element incorporated diamond films. Carbon 2021, 178, 19–25. [Google Scholar] [CrossRef]
- Mahmoudi Alemi, F.; Mohammadi, S.; Mousavi Dehghani, S.A.; Rashidi, A.; Hosseinpour, N.; Seif, A. Experimental and DFT studies on the effect of carbon nanoparticles on asphaltene precipitation and aggregation phenomena. Chem. Eng. J. 2021, 422, 130030. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y.; Luo, B. Photocatalytic degradation of methyl orange by Ag/TiO2/biochar composite catalysts in aqueous solutions. Mat. Sci. Semicond. Proc. 2020, 114, 105088. [Google Scholar] [CrossRef]
- Ramasahayam, S.K.; Nasini, U.B.; Shaikh, A.U.; Viswanathan, T. Novel tannin-based Si, P co-doped carbon for supercapacitor applications. J. Power Sources 2015, 275, 835–844. [Google Scholar] [CrossRef]











| Item | Properties |
|---|---|
| Coating method | Physical Vapor Deposition (PVD) |
| Carbon source | High-purity graphite |
| Transition layer | Cr interlayer |
| Thickness (μm) | 3.0 |
| Surface roughness, Ra (nm) | 5.9 ± 0.6 |
| Hardness (GPa) | 10.6 ± 0.4 |
| Young Modulus (GPa) | 160.6 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Ding, Q.; Gu, Y.; Quan, X.; Ma, Y.; Jia, Y.; Xie, H.; Tang, J. Study of Tribological Properties of Fullerenol and Nanodiamonds as Additives in Water-Based Lubricants for Amorphous Carbon (a-C) Coatings. Nanomaterials 2022, 12, 139. https://doi.org/10.3390/nano12010139
Chen S, Ding Q, Gu Y, Quan X, Ma Y, Jia Y, Xie H, Tang J. Study of Tribological Properties of Fullerenol and Nanodiamonds as Additives in Water-Based Lubricants for Amorphous Carbon (a-C) Coatings. Nanomaterials. 2022; 12(1):139. https://doi.org/10.3390/nano12010139
Chicago/Turabian StyleChen, Shuqing, Qi Ding, Yan Gu, Xin Quan, Ying Ma, Yulong Jia, Hongmei Xie, and Jinzhu Tang. 2022. "Study of Tribological Properties of Fullerenol and Nanodiamonds as Additives in Water-Based Lubricants for Amorphous Carbon (a-C) Coatings" Nanomaterials 12, no. 1: 139. https://doi.org/10.3390/nano12010139