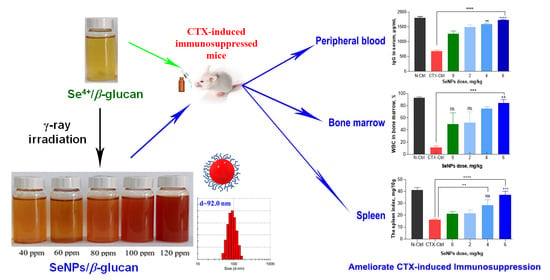
Radiation Synthesis of Selenium Nanoparticles Capped with β-Glucan and Its Immunostimulant Activity in Cytoxan-Induced Immunosuppressed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SeNPs/β-Glucan by Gamma Co-60 Irradiation
2.3. Preparation of SeNPs/β-Glucan Powder
2.4. Analysis of Se4+ in Irradiated Samples
2.5. Characterization of SeNPs/β-Glucan
2.6. Animal Experimental Design
2.7. Spleen Index Determination
2.8. Analysis of Cytokine and Immunoglobulin Indexes in Serum and Spleen
2.9. Determination of Cellular Immunity
3. Results and Discussion
3.1. Characteristics of Radiation Synthesized SeNPs
3.2. Effect of SeNPs/β-Glucan on Immune Parameters in Peripheral Blood
3.3. Effect of SeNPs/β-Glucan on Immune Parameters in Bone Marrow
3.4. Effect of SeNPs/β-Glucan on Immune Parameters in Spleen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BSA | bovine serum albumin |
| CTX | cytoxan |
| DLS | dynamic light scattering |
| FBS | fetal bovine serum |
| FTIR | Fourier transform infrared spectroscopy |
| IL-2 | interleukin 2 |
| IFN-γ | interferon-γ |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| LSD | least significant difference |
| NK cell | natural killer cell |
| PBS | phosphate buffered saline |
| SeNPs | selenium nanoparticles |
| TEM | transmission electron microscopy |
| TNF-α | tumor necrosis factor alpha |
| WBC | white blood cell |
| XRD | X-ray diffraction |
References
- Beck, M.A.; Levander, O.A.; Handy, J. Selenium deficiency and viral infection. J. Nutr. 2003, 133, 1463S–1467S. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Zeng, H.; Combs, G.F.J. Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008, 19, 1–7. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Wang, C.R.C. Evidence on the size-dependent absorption spectral evolution of selenium nanoparticles. Mater. Chem. Phys. 2005, 92, 591–594. [Google Scholar] [CrossRef]
- Yang, L.B.; Shen, Y.H.; Xie, A.J.; Liang, J.J.; Zhang, B.C. Synthesis of Se nanoparticles by using TSA ion and its photocatalytic application for decolorization of cango red under UV irradiation. Mater. Res. Bull. 2008, 43, 572–582. [Google Scholar] [CrossRef]
- Raevskaya, A.E.; Stroyuk, A.L.; Kuchmiy, S.Y.; Dzhagan, V.M.; Zahn, D.R.T.; Schulze, S. Annealing-induced structural transformation of gelatin-capped Se nanoparticles. Solid State Commun. 2008, 145, 288–292. [Google Scholar] [CrossRef]
- Hien, N.Q.; Tuan, P.D.; Van Phu, D.; Lan, N.T.K.; Duy, N.N.; Hoa, T.T. Gamma Co-60 ray irradiation synthesis of dextran stabilized selenium nanoparticles and their antioxidant activity. Mater. Chem. Phys. 2018, 205, 29–34. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of short-term toxicity between nano-Se and selenite in mice. Life Sci. 2005, 76, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Pelyhe, C.; Mezes, M. Myths and facts about the effects of nano-selenium in farm animals- mini review. Eur. Chem. Bull. 2013, 2, 1049–1052. [Google Scholar]
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Zhai, X.; Zhang, C.; Zhao, S.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 4. [Google Scholar] [CrossRef] [Green Version]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdi, M.H.; Mahdavi, M.; Varastehmoradi, B.; Faramarzi, M.A.; Shahverdi, A.R. The immunostimulatory effect of biogenic selenium nanoparticles on the 4T1 breast cancer model: an in vivo study. Biol. Trace. Elem. Res. 2012, 149, 22–28. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Mahdavi, M.; Faghfuri, E.; Faramarzi, M.A.; Sepehrizadeh, Z.; Hassan, Z.M.; Gholami, M.; Shahverdi, A.R. Th1 immune response induction by biogenic selenium nanoparticles in mice with breast cancer: preliminary vaccine model. Iran J. Biotechnol. 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Liu, Q.; Zou, S.; Xu, X.; Zhang, L. Construction of selenium nanoparticles/β-glucan composites for enhancement of the antitumor activity. Carbohydr. Polym. 2015, 117, 434–442. [Google Scholar] [CrossRef]
- Duy, N.N.; Phu, D.V.; Quoc, L.A.; Lan, N.T.K.; Hien, N.Q.; Ngan, T.T.T.; Ha, T.L.B.; Tuan, P.D.; Ha, B.M. Preparation and effect of selenium nanoparticles/oligochitosan on the white blood cell recovery of mice exposed to gamma-ray radiation. J. Chem. 2021, 2021, 6635022. [Google Scholar]
- Menon, S.; Ks, S.D.; Santhiya, R.; Rajeshkumar, S.; Kumar, V. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B Biointerfaces 2018, 170, 280–292. [Google Scholar] [CrossRef]
- Kumari, M.; Ray, L.; Purohit, M.P.; Patnaik, S.; Pant, A.B.; Shukla, Y.; Kumar, P.; Gupta, K.C. Curcumin loading potentiates the chemotherapeutic efficacy of selenium nanoparticles in HCT116 cells and Ehrlich’s ascites carcinoma bearing mice. Eur. J. Pharm. Biopharm. 2017, 117, 346–362. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.G.; Zhao, K.; Sun, D.; Li, X.; Wan, J. Inverse relationship between elemental selenium nanoparticle size and inhibition of cancer cell growth in vitro and in vivo. Food Chem. Toxicol. 2015, 85, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, J.S.; Liu, Q.L.; Ethan, W.T. Size effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J. Inorg. Biochem. 2007, 101, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, J.; Hou, J.; Chen, C. Free radical scavenging efficiency of Nano-Se in vitro. Free Radical Biol. Med. 2003, 35, 805–813. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Xu, Y.L.; Bai, Y.; Deng, Y.; Liu, J.; Chen, L. Green synthesis of Se/Ru alloy nanoparticles using gallic acid and evaluation of theiranti-invasive effects in HeLa cells. Colloids Surf. B Biointerfaces 2016, 144, 118–124. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, L.; Jiang, W.; Fu, Y.; Zheng, W.; Chen, T. Rational design of cancertargeted selenium nanoparticles to antagonize multidrug resistance in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Zhang, Y.; Zheng, W. Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int. J. Nanomed. 2012, 7, 835–844. [Google Scholar]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhai, X.; Zhao, G.; Ren, F.; Leng, X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr. Polym. 2015, 134, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Zechner-krpan, V.; Petravić-tominac, V.; Panjkota-krbavčić, I.; Grba, S.; Berković, K. Potential application of yeast β-Glucans in food industry. Agric. Conspec. Sci. 2009, 74, 277–282. [Google Scholar]
- Suzuki, T.; Tanaka, H.; Kinoshita, A.; Oikawa, S.; Osawa, M.; Yadomae, T. Effect of orally administered beta-glucan in macrophage function in mice. Int. J. Immunopharmacol. 1990, 12, 675–684. [Google Scholar] [CrossRef]
- Reed, G.; Nagodawithana, T.W. Yeast-derived products and food and feed yeast. In Yeast Technology; Van Nostrand Reinhold: New York, NY, USA, 1991. [Google Scholar]
- Sung, N.Y.; Byun, E.H.; Kwon, S.K.; Song, B.S.; Choi, J.I.; Kim, J.H.; Byun, M.W.; Yoo, Y.C.; Kim, M.R.; Lee, J.W. Immune enhancing activities of low molecular weight β-glucan depolymerized by gamma irradiation. Rad. Phys. Chem. 2009, 78, 433–436. [Google Scholar] [CrossRef]
- Sung, N.Y.; Choi, J.; Yoon, Y.; Lee, S.Y.; Byun, M.W.; Hwang, Y.J.; Koenari, Z.I.; Lee, J.W.; Kim, J.H. Anti-allergic Effect of Low Molecular Weight β-Glucan Prepared by γ-Irradiation. Food Sci. Biotechnol. 2011, 20, 841–844. [Google Scholar] [CrossRef]
- Lee, J.W.; Byun, E.H.; Sung, N.Y.; Raghavendran, H.R.B.; Byun, E.B.; Kim, J.H.; Choi, J.; Shin, M.G.; Byun, M.W. Effect of gamma irradiation on the efficacy of β-glucan against acetaminophen induced toxicity in mice. Chem. Biol. Interact. 2009, 180, 98–105. [Google Scholar] [CrossRef]
- Long, N.T.; Anh, N.; Giang, B.L.; Son, H.N.; Luan, L.Q. Radiation Degradation of β-Glucan with a Potential for Reduction of Lipids and Glucose in the Blood of Mice. Polymers 2019, 11, 955. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M.; Nayarana, B. An easy spectrophotometric determination of selenium using azure B as a chromogenic reagent. Indian J. Chem. Technol. 2006, 13, 155–158. [Google Scholar]
- Guo, M.Z.; Meng, M.; Feng, C.C.; Wang, X.; Wang, C.L. A novel polysaccharide obtained from Craterellus cornucopioides enhances immunomodulatory activity in immunosuppressive mice models via regulation of the TLR4-NF-κB pathway. Food Funct. 2019, 10, 4792–4801. [Google Scholar] [CrossRef]
- Han, L.; Meng, M.; Guo, M.; Cheng, D.; Shi, L.; Wang, X.; Wang, C. Immunomodulatory activity of a water-soluble polysaccharide obtained from highland barley on immunosuppressive mice models. Food Funct. 2018, 10, 304–314. [Google Scholar] [CrossRef]
- Hien, N.Q.; Phu, D.V.; Duy, N.N.; Quoc, L.A. Radiation synthesis and characterization of hyaluronan capped gold nanoparticles. Carbohydr. Polym. 2012, 89, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Khoa, N.D.; Christelle, K.; Laurent, D.; Xavier, C.; Hien, N.Q. Radiation synthesis of chitosan stabilized gold nanoparticles comparison between e- beam and γ irradiation. Rad. Phys. Chem. 2014, 94, 84–87. [Google Scholar]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. 2016, 733, 012039. [Google Scholar] [CrossRef] [Green Version]
- Miguel, A.; Ruiz, F.; Jsemaría, D.M.; Jaime, G.B.; María, V.F.C.; Germán, B.E.; Marcos, F.M.M.; Mohamed, L.M. Green synthesis and biotransformation of amorphous Se nanospheres to trigonal 1D Se nanostructures: impact on Se mobility within the concept of radioactive waste disposal. Environ. Sci. Nano 2018, 5, 2103–2116. [Google Scholar]
- Menazeaa, A.A.; Ismail, A.M.; Nasser, S.A.; Hala, A.I. Physical characterization and antibacterial activity of VA/Chitosan matrix doped by selenium nanoparticles prepared via one-pot laser ablation route. J. Mater. Res. Technol. 2020, 9, 9598–9606. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Ferrante, F.; Cavallaro, G.; Alduina, R.; Martino, D.F.C. Biogenic Selenium Nanoparticles: A Fine Characterization to Unveil Their Thermodynamic Stability. Nanomaterials 2021, 11, 1195. [Google Scholar] [CrossRef]
- Thao, D.T.T.; Phu, D.V.; Duy, N.N.; Quy, H.T.D.; Hoa, T.T.; Hien, N.Q. Synthesis of gold nanoparticles stabilized in dextran solution by gamma Co-60 ray irradiation and preparation of gold nanoparticles/dextran powder. J. Chem. 2017, 2017, 1–8. [Google Scholar]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Zhang, W.N.; Gong, L.L.; Liu, Y.; Zhou, Z.B.; Wan, C.X.; Xu, J.J.; Chen, Y. Immunoenhancement effect of crude polysaccharides of Helvella leucopus on cyclophosphamide-induced immunosuppressive mice. J. Funct. Foods 2020, 69, 103942. [Google Scholar] [CrossRef]
- Gao, F.; Yuan, Q.; Gao, L.; Cai, P.; Zhu, H.; Liu, R.; Wang, Y.; Wei, Y.; Huang, J.G.; Liang, X. Cytotoxicity and therapeutic effect of irinotecan combined with selenium nanoparticles. Biomaterials 2014, 35, 8854–8866. [Google Scholar] [CrossRef]
- Shakibaie, M.; Shahverdi, A.R.; Faramarzi, M.A.; Hassanzadeh, G.R.; Rahimi, H.R.; Sabzevari, O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm. Biol. 2012, 51, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.H.; Masoudifar, M.; Varastehmoradi, B.; Mohammadi, E.; Kheradmand, E.; Homayouni, S.; Shahverdi, A.R. Effect of oral supplementation of biogenic selenium nanoparticles on white blood cell profile of BALB/c mice and mice exposed to X-ray radiation. Avicenna J. Med. Biotechnol. 2013, 5, 158–167. [Google Scholar] [PubMed]
- Raahati, Z.; Bakhshi, B.; Najar-peerayeh, S. Selenium Nanoparticles Induce Potent Protective Immune Responses against Vibrio cholerae WC Vaccine in a Mouse Model. J. Immunol. Res. 2020, 2020, 8874288. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Bao, Y.; Zhang, L. Nano red elemental selenium has no size effect in the induction of seleno-enzymes in both cultured cells and mice. Life Sci. 2004, 75, 237–244. [Google Scholar] [CrossRef]
- Arslan, S.; Ozyurek, E.; Gunduz-Demir, C. A color and shape based algorithm for segmentation of white blood cells in peripheral bood and bone marrow images. Cytom. A 2014, 85, 480–490. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dung, N.T.; Trong, T.D.; Vu, N.T.; Binh, N.T.; Minh, T.T.L.; Luan, L.Q. Radiation Synthesis of Selenium Nanoparticles Capped with β-Glucan and Its Immunostimulant Activity in Cytoxan-Induced Immunosuppressed Mice. Nanomaterials 2021, 11, 2439. https://doi.org/10.3390/nano11092439
Dung NT, Trong TD, Vu NT, Binh NT, Minh TTL, Luan LQ. Radiation Synthesis of Selenium Nanoparticles Capped with β-Glucan and Its Immunostimulant Activity in Cytoxan-Induced Immunosuppressed Mice. Nanomaterials. 2021; 11(9):2439. https://doi.org/10.3390/nano11092439
Chicago/Turabian StyleDung, Nguyen Thi, Tran Duc Trong, Nguyen Thanh Vu, Nguyen Trong Binh, Tran Thi Le Minh, and Le Quang Luan. 2021. "Radiation Synthesis of Selenium Nanoparticles Capped with β-Glucan and Its Immunostimulant Activity in Cytoxan-Induced Immunosuppressed Mice" Nanomaterials 11, no. 9: 2439. https://doi.org/10.3390/nano11092439