A Comparison between Silver Nanosquare Arrays and Silver Thin-Films as a Blood Cancer Prognosis Monitoring Electrode Design Using Optical and Electrochemical Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
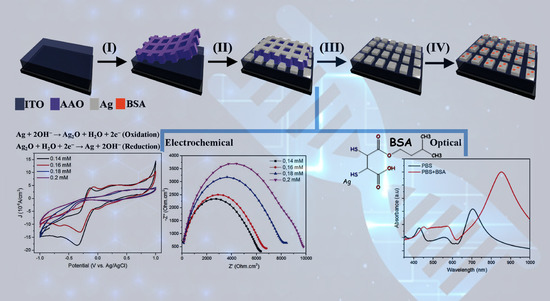
2.2. Fabrication of AAO
2.3. Ag Deposition
2.4. Ag Characterization
2.5. Simulation Session
3. Results and Discussion
3.1. Structure Analysis
3.2. Electric Field Distribution and Optical Character Analysis
3.3. Electrochemical Analysis of Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Gole, J.; Gore, A.; He, Q.; Lu, M.; Min, J.; Yuan, Z.; Yang, X.; Jiang, Y.; Zhang, T.; et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat. Commun. 2020, 11, 3475. [Google Scholar] [CrossRef]
- Hawkes, N. Cancer survival data emphasise importance of early diagnosis. BMJ 2019, 364, l408. [Google Scholar] [CrossRef]
- Agrawal, R.; Satapathy, S.; Bagla, G.; Rajakumar, K. Detection of White Blood Cell Cancer using Image Processing. In Proceedings of the 2019 International Conference on Vision Towards Emerging Trends in Communication and Networking (ViTECoN), Vellore, India, 30–31 March 2019; pp. 1–6. [Google Scholar]
- Benedetti, E.; Bramanti, E.; Papineschi, F.; Rossi, I.; Benedetti, E. Determination of the Relative Amount of Nucleic Acids and Proteins in Leukemic and Normal Lymphocytes by Means of Fourier Transform Infrared Microspectroscopy. Appl. Spectrosc. 1997, 51, 792–797. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Yu, X.; Chen, D.; Liu, A. Diagnosis of human malignancies using laser-induced breakdown spectroscopy in combination with chemometric methods. Spectrochim. Acta Part B At. Spectrosc. 2018, 139, 63–69. [Google Scholar] [CrossRef]
- Prabhakar, S.; Jain, N.; Singh, R.A. FT-IR Spectroscopy as a Bio-Diagnostic Tool for Detection of Leukemia. In Emerging Trends in Laser & Spectroscopy and Applications; Allied Publishers: New Delhi, India, 2013; p. 5. ISBN 978-81-8424-626-1. [Google Scholar]
- Webster, J.G. Medical Instrumentation Application and Design, 4th ed.; John Wiley & Sons, Incorporated: Madison, WI, USA, 2009; ISBN 978-1-118-31285-8. [Google Scholar]
- Luka, G.; Samiei, E.; Dehghani, S.; Johnson, T.; Najjaran, H.; Hoorfar, M. Label-Free Capacitive Biosensor for Detection of Cryptosporidium. Sensors 2019, 19, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabin, A.; Ahmed, K.; Rana, J.; Paul, B.K.; Islam, M.; Vigneswaran, D.; Uddin, M.S. Surface Plasmon Resonance Based Titanium Coated Biosensor for Cancer Cell Detection. IEEE Photonics J. 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Goossens, N.; Nakagawa, S.; Sun, X.; Hoshida, Y. Cancer biomarker discovery and validation. Transl. Cancer Res. 2015, 4, 256–269. [Google Scholar] [PubMed]
- Filliatre-Clement, L.; Broseus, J.; Muller, M.; Hosseini, K.; Rotonda, C.; Schirmer, L.; Roth-Guepin, G.; Bonmati, C.; Feugier, P.; Béné, M.; et al. Serum albumin or body mass index: Which prognostic factor for survival in patients with acute myeloblastic leukaemia? Hematol. Oncol. 2019, 37, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Sadek, N.A.; Abd-Eltawab, S.M.; Assem, N.M.; Hamdy, H.A.-M.; EL-sayed, F.M.; Ahmad, M.A.-R.; Elhamammy, R.H. Prognostic Value of Absolute Lymphocyte count, Lymphocyte percentage, Serum albumin, Aberrant Expression of CD7, CD19 and the Tumor Suppressors (PTEN and p53) in Patients with Acute Myeloid Leukemia. Asian Pac. J. Cancer Biol. 2020, 5, 131–140. [Google Scholar] [CrossRef]
- Ertürk, G.; Berillo, D.; Hedström, M.; Mattiasson, B. Microcontact-BSA imprinted capacitive biosensor for real-time, sensitive and selective detection of BSA. Biotechnol. Rep. 2014, 3, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zheng, X.; Xing, W.; Huang, J.; Li, G. Electrochemical Studies of Camptothecin and Its Interaction with Human Serum Albumin. Int. J. Mol. Sci. 2007, 8, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W. A label-free biosensor based on silver nanoparticles array for clinical detection of serum p53 in head and neck squamous cell carcinoma. Int. J. Nanomed. 2011, 6, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Yazdanparast, S.; Benvidi, A.; Banaei, M.; Nikukar, H.; Tezerjani, M.D.; Azimzadeh, M. Dual-aptamer based electrochemical sandwich biosensor for MCF-7 human breast cancer cells using silver nanoparticle labels and a poly(glutamic acid)/MWNT nanocomposite. Microchim. Acta 2018, 185, 405. [Google Scholar] [CrossRef]
- Pothipor, C.; Wiriyakun, N.; Putnin, T.; Ngamaroonchote, A.; Jakmunee, J.; Ounnunkad, K.; Laocharoensuk, R.; Aroonyadet, N. Highly sensitive biosensor based on graphene–poly (3-aminobenzoic acid) modified electrodes and porous-hollowed-silver-gold nanoparticle labelling for prostate cancer detection. Sens. Actuators B Chem. 2019, 296, 126657. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Vivekanandan, S. A comprehensive review on thin film-based nano-biosensor for uric acid determination: Arthritis diagnosis. World Rev. Sci. Technol. Sustain. Dev. 2018, 14, 52. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Kwak, J.-W.; Park, J.W. Nanotechnology for Early Cancer Detection. Sensors 2010, 10, 428–455. [Google Scholar] [CrossRef]
- Velasco-Rodriguez, B.; Soltero-Martínez, J.F.; Rosales-Rivera, L.C.; Macías-Balleza, E.R.; Landázuri, G.; Larios-Durán, E.R. Adsorption and Interaction of Bovine Serum Albumin and Pluronic P103 Triblock Copolymer on a Gold Electrode: Double-Layer Capacitance Measurements. ACS Omega 2020, 5, 17347–17355. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-Y.; Xu, J.-J.; Wang, Z.-H.; Chen, H.-Y. A novel signal-amplified electrochemical aptasensor based on supersandwich G-quadruplex DNAzyme for highly sensitive cancer cell detection. Electrochem. Commun. 2015, 52, 49–52. [Google Scholar] [CrossRef]
- Bábelová, L.; Slabý, C.; Hianik, T. The Development of Electrochemical Aptasensor Based on DNA Aptamers Modified by Redox Markers for Detection of Leukemia Jurkat Cells. In Proceedings of the 1st International Electronic Conference on Biosensors, Online, 2–17 November 2020. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L. Comparative study of Ag and Au nanoparticles biosensors based on surface plasmon resonance phenomenon. Mater. Sci. Eng. C 2012, 32, 1437–1442. [Google Scholar] [CrossRef]
- Xu, P.J.; Liu, H.; Zhang, H.; Tao, X.M.; Wang, S.Y. Electrochemical Modification of Silver Coated Multifilament for Wearable ECG Monitoring Electrodes. Adv. Mater. Res. 2011, 332–334, 1019–1023. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Cumbo, C.; Anelli, L.; Specchia, G.; Albano, F. Monitoring of Minimal Residual Disease (MRD) in Chronic Myeloid Leukemia: Recent Advances. Cancer Manag. Res. 2020, 12, 3175–3189. [Google Scholar] [CrossRef]
- Hianik, T. Advances in Electrochemical and Acoustic Aptamer-Based Biosensors and Immunosensors in Diagnostics of Leukemia. Biosensors 2021, 11, 177. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Tsai, M.-S. Tunable Silver Nanoparticle Arrays by Hot Embossing and Sputter Deposition for Surface-Enhanced Raman Scattering. Appl. Sci. 2019, 9, 1636. [Google Scholar] [CrossRef] [Green Version]
- Losic, D.; Velleman, L.; Kant, K.; Kumeria, T.; Gulati, K.; Shapter, J.; Beattie, D.A.; Simovic, S. Self-ordering Electrochemistry: A Simple Approach for Engineering Nanopore and Nanotube Arrays for Emerging Applications. Aust. J. Chem. 2011, 64, 294–301. [Google Scholar] [CrossRef]
- Nasori, N.; Cao, D.; Wang, Z.; Farahdina, U.; Rubiyanto, A.; Lei, Y. Tunning of Templated CuWO4 Nanorods Arrays Thickness to Improve Photoanode Water Splitting. Molecules 2021, 26, 2900. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Sydoruk, O.; Zhernovaya, O.; Tuchin, V.; Douplik, A. Refractive index of solutions of human hemoglobin from the near-infrared to the ultraviolet range: Kramers-Kronig analysis. J. Biomed. Opt. 2012, 17, 115002. [Google Scholar] [CrossRef]
- Banerjee, A.; Maity, S.; Mastrangelo, C. Nanostructures for Biosensing, with a Brief Overview on Cancer Detection, IoT, and the Role of Machine Learning in Smart Biosensors. Sensors 2021, 21, 1253. [Google Scholar] [CrossRef]
- Parsianpour, E.; Raoufi, D.; Roostaei, M.; Sohrabi, B.; Samavat, F. Characterization and Structural Property of Indium Tin Oxide Thin Films. Adv. Mater. Phys. Chem. 2017, 7, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Beranek, R.; Tsuchiya, H.; Sugishima, T.; Macak, J.; Taveira, L.V.; Fujimoto, S.; Kisch, H.; Schmuki, P. Enhancement and limits of the photoelectrochemical response from anodic TiO2 nanotubes. Appl. Phys. Lett. 2005, 87, 243114. [Google Scholar] [CrossRef]
- Fajar, M.N.; Endarko, E.; Rubiyanto, A.; Malek, N.A.N.N.; Hadibarata, T.; Syafiuddin, A. A green deposition method of silver nanoparticles on textiles and their antifungal activity. Biointerface Res. Appl. Chem. 2019, 10, 4902–4907. [Google Scholar] [CrossRef]
- Fitzpatrick, R. Maxwell’s Equations and the Principles of Electromagnetism; Jones & Bartlett Publishers: Burlington, MA, USA, 2008; ISBN 978-1-934015-20-9. [Google Scholar]
- Di Barba, P.; Savini, A.; Wiak, S. Field Models in Electricity and Magnetism; Springer Science & Business Media: Zurich, Switzerland, 2008; ISBN 978-1-4020-6843-0. [Google Scholar]
- Ma, W.; Shi, T.; Tang, Z.; Liu, S.; Malik, R.; Zhang, L. High-throughput dielectrophoretic manipulation of bioparticles within fluids through biocompatible three-dimensional microelectrode array. Electrophoresis 2011, 32, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.P. Semiconductors: An Introduction. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2002; pp. 1–16. ISBN 978-0-08-043152-9. [Google Scholar]
- Wu, C.; Liang, W.; Fa, J.; Cao, Y.; Wu, P.; Cai, C. Regulating Electron Transport Band Gaps of Bovine Serum Albumin by Binding Hemin. Acta Physico-Chim. Sin. 2020, 37, 1912050. [Google Scholar] [CrossRef]
- Englebienne, P. Use of colloidal gold surface plasmon resonance peak shift to infer affinity constants from the interactions between protein antigens and antibodies specific for single or multiple epitopes. Analyst 1998, 123, 1599–1603. [Google Scholar] [CrossRef]
- Nonoyama, A. Using Multiwavelength UV-Visible Spectroscopy for the Characterization of Red Blood Cells: An Investigation of Hypochromism. Ph.D. Theses, University of South Florida, Tampa, FL, USA, 2004. [Google Scholar]
- De Jonge, H.J.M.; Valk, P.J.M.; De Bont, E.S.J.M.; Schuringa, J.J.; Ossenkoppele, G.; Vellenga, E.; Huls, G. Prognostic impact of white blood cell count in intermediate risk acute myeloid leukemia: Relevance of mutated NPM1 and FLT3-ITD. Haematologica 2011, 96, 1310–1317. [Google Scholar] [CrossRef]
- Wang, N.; Desai, A.; Ge, B.; Li, W.; Jin, X.; Bai, H.; Yu, K.; Ye, H. Prognostic value of hypoalbuminemia at diagnosis in de novo non-M3 acute myeloid leukemia. Leuk. Lymphoma 2020, 61, 641–649. [Google Scholar] [CrossRef]
- Luo, H.; Ji, X.; Cheng, S. Investigation into the electrochemical behaviour of silver in alkaline solution and the influence of Au-decoration using operando Raman spectroscopy. RSC Adv. 2020, 10, 8453–8459. [Google Scholar] [CrossRef] [Green Version]
- Oje, A.I.; Ogwu, A.; Mirzaeian, M.; Tsendzughul, N. Electrochemical energy storage of silver and silver oxide thin films in an aqueous NaCl electrolyte. J. Electroanal. Chem. 2018, 829, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Gu, Y.; Zhang, F.; Liu, Z.; Li, Y.; Chen, G.; Wang, B. High-Performance Non-enzymatic Glucose Sensors Based on CoNiCu Alloy Nanotubes Arrays Prepared by Electrodeposition. Front. Mater. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-X.; Bao, N.; Luo, X.; Ding, S.-N. Nonenzymatic Amperometric Aptamer Cytosensor for Ultrasensitive Detection of Circulating Tumor Cells and Dynamic Evaluation of Cell Surface N-Glycan Expression. ACS Omega 2018, 3, 8595–8604. [Google Scholar] [CrossRef] [PubMed]
- Ciribelli, B.N.; Colmati, F.; De Souza, E.C. Nernst equation applied to electrochemical systems and centenary of his Nobel Prize in chemistry. Int. J. Innov. Educ. Res. 2020, 8, 670–683. [Google Scholar] [CrossRef]
- Wang, S.X.; Acha, D.; Shah, A.J.; Hills, F.; Roitt, I.; Demosthenous, A.; Bayford, R.H. Detection of the tau protein in human serum by a sensitive four-electrode electrochemical biosensor. Biosens. Bioelectron. 2017, 92, 482–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushworth, J.V.; Ahmed, A.; Jarosz-Griffiths, H.; Pollock, N.M.; Hooper, N.; Millner, P.A. A label-free electrical impedimetric biosensor for the specific detection of Alzheimer’s amyloid-beta oligomers. Biosens. Bioelectron. 2014, 56, 83–90. [Google Scholar] [CrossRef]
- Rains, M.K.; Martić, S.; Freeman, D.; Kraatz, H.B. Electrochemical Investigations into Kinase-Catalyzed Transformations of Tau Protein. ACS Chem. Neurosci. 2013, 4, 1194–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasori, N.; Farahdina, U.; Zulfa, V.Z.; Firdhaus, M.; Aziz, I.; Darsono, D.; Cao, D.; Wang, Z.; Endarko, E.; Rubiyanto, A. A Comparison between Silver Nanosquare Arrays and Silver Thin-Films as a Blood Cancer Prognosis Monitoring Electrode Design Using Optical and Electrochemical Characterization. Nanomaterials 2021, 11, 3108. https://doi.org/10.3390/nano11113108
Nasori N, Farahdina U, Zulfa VZ, Firdhaus M, Aziz I, Darsono D, Cao D, Wang Z, Endarko E, Rubiyanto A. A Comparison between Silver Nanosquare Arrays and Silver Thin-Films as a Blood Cancer Prognosis Monitoring Electrode Design Using Optical and Electrochemical Characterization. Nanomaterials. 2021; 11(11):3108. https://doi.org/10.3390/nano11113108
Chicago/Turabian StyleNasori, Nasori, Ulya Farahdina, Vinda Zakiyatuz Zulfa, Miftakhul Firdhaus, Ihwanul Aziz, Darsono Darsono, Dawei Cao, Zhijie Wang, Endarko Endarko, and Agus Rubiyanto. 2021. "A Comparison between Silver Nanosquare Arrays and Silver Thin-Films as a Blood Cancer Prognosis Monitoring Electrode Design Using Optical and Electrochemical Characterization" Nanomaterials 11, no. 11: 3108. https://doi.org/10.3390/nano11113108