Effects of Process Variables on Properties of CoFe2O4 Nanoparticles Prepared by Solvothermal Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Cobalt Ferrite Nanoparticles
2.3. Characterization
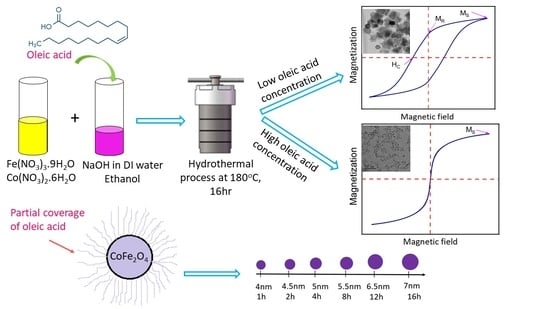
3. Results
3.1. Effect of Reaction Time on the Morphology of CoFe2O4 Nanoparticles
3.2. Effect of Reaction Temperature on the Morphology of CoFe2O4 Nanoparticles
3.3. Effect of Oleic Acid Concentration on the Morphology of CoFe2O4 Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatima, H.; Charinpanitkul, T.; Kim, K.-S. Fundamentals to apply magnetic nanoparticles for hyperthermia therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kim, K.S. Controlled magnetic properties of iron oxide-based nanoparticles for smart therapy. KONA Powder Part. J. 2016, 2016, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Fatima, H.; Kim, K.S. Magnetic nanoparticles for bioseparation. Korean J. Chem. Eng. 2017, 34, 589–599. [Google Scholar] [CrossRef]
- Tang, S.Q.; Moon, S.J.; Park, K.H.; Paek, S.H.; Chung, K.W.; Bae, S. Feasibility of TEOS coated CoFe2O4 nanoparticles to a GMR biosensor agent for single molecular detection. J. Nanosci. Nanotechnol. 2011, 11, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Gyergyek, S.; Drofenik, M.; Makovec, D. Oleic-acid-coated CoFe2O4 nanoparticles synthesized by co-precipitation and hydrothermal synthesis. Mater. Chem. Phys. 2012, 133, 515–522. [Google Scholar] [CrossRef]
- Srinivasan, S.Y.; Paknikar, K.M.; Bodas, D.; Gajbhiye, V. Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine 2018, 13, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Gandha, K.; Elkins, K.; Poudyal, N.; Liu, J.P. Synthesis and characterization of CoFe2O4 nanoparticles with high coercivity. J. Appl. Phys. 2015, 117, 17. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Ghanbari, D.; Salavati-Niasari, M. Photoluminescence carbon dot as a sensor for detecting of Pseudomonas aeruginosa bacteria: Hydrothermal synthesis of magnetic hollow NiFe2O4 -carbon dots nanocomposite material. Compos. Part B Eng. 2019, 161, 564–577. [Google Scholar] [CrossRef]
- Joulaei, M.; Hedayati, K.; Ghanbari, D. Investigation of magnetic, mechanical and flame retardant properties of polymeric nanocomposites: Green synthesis of MgFe2O4 by lime and orange extracts. Compos. Part B Eng. 2019, 176, 107345. [Google Scholar] [CrossRef]
- Masoumi, S.; Nabiyouni, G.; Ghanbari, D. Photo-degradation of Congored, acid brown and acid violet: Photo catalyst and magnetic investigation of CuFe2O4 –TiO2 –Ag nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 27, 11017–11033. [Google Scholar] [CrossRef]
- Jang, J.T.; Nah, H.; Lee, J.H.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.Y.; Andújar, M.S.; Aguirre, C.G.; Mira, J.; Rodríguez, M.A.S.; García, S.C. A simple solvothermal synthesis of MFe2O4 (M=Mn, Co and Ni) nanoparticles. J. Solid State Chem. 2009, 182, 2685–2690. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Vilar, S.; Sánchez-Andújar, M.; Gómez-Aguirre, C.; Mira, J.; Señarís-Rodríguez, M.A.; Castro-García, S. A simple solvothermal synthesis of MFe2O4 (M=Mn, Co and Ni) nanoparticles. J. Solid State Chem. 2009, 182, 2685–2690. [Google Scholar] [CrossRef] [Green Version]
- Munjal, S.; Khare, N.; Sivakumar, B.; Sakthikumar, D.N. Citric acid coated CoFe2O4 nanoparticles transformed through rapid mechanochemical ligand exchange for efficient magnetic hyperthermia applications. J. Magn. Magn. Mater. 2019, 477, 388–395. [Google Scholar] [CrossRef]
- Kazemi, M.; Ghobadi, M.; Mirzaie, A. Cobalt ferrite nanoparticles (CoFe2O4 MNPs) as catalyst and support: Magnetically recoverable nanocatalysts in organic synthesis. Nanotechnol. Rev. 2018, 7, 43–68. [Google Scholar] [CrossRef]
- Wu, L.; Jubert, P.; Berman, D.; Imaino, W.; Nelson, A.; Zhu, H.; Zhang, S.; Sun, S. Monolayer assembly of ferrimagnetic CoxFe3-xO4 nanocubes for magnetic recording. Nano Lett. 2014, 14, 3395–3399. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Sun, S. Chemical synthesis of magnetic nanoparticles for permanent magnet applications. Chem.-A Eur. J. 2020, 26, 6757–6766. [Google Scholar] [CrossRef] [PubMed]
- Bortnic, R.; Goga, F.; Mesaroş, A.; Nasui, M.; Vasile, B.S.; Roxana, D.; Avram, A. Synthesis of cobalt ferrite nanoparticles via a sol-gel combustion method. Stud. Ubb Chem. 2016, 4, 213–222. [Google Scholar]
- Tomar, D.; Jeevanandam, P. Synthesis of cobalt ferrite nanoparticles with different morphologies via thermal decomposition approach and studies on their magnetic properties. J. Alloys Compd. 2020, 843, 155815. [Google Scholar] [CrossRef]
- Foroughi, F.; Hassanzadeh-Tabrizi, S.A.; Amighian, J. Microemulsion synthesis and magnetic properties of hydroxyapatite-encapsulated nano CoFe2O4. J. Magn. Magn. Mater. 2015, 382, 182–187. [Google Scholar] [CrossRef]
- Kalam, A.; Al-Sehemi, A.G.; Assiri, M.; Du, G.; Ahmad, T.; Ahmada, I.; Pannipara, M. Modified solvothermal synthesis of cobalt ferrite (CoFe2O4) magnetic nanoparticles photocatalysts for degradation of methylene blue with H2O2 visible light. Results Phys. 2018, 8, 1046–1053. [Google Scholar] [CrossRef]
- Shanmugam, S.; Subramanian, B. Evolution of phase pure magnetic cobalt ferrite nanoparticles by varying the synthesis conditions of polyol method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 252, 114451. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Recent advances in synthesis and applications of MFe2O4 (M = Co, Cu, Mn, Ni, Zn) nanoparticles. J. Mater. 2021, 4, 1560. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allaedini, G.; Tasirin, S.M.; Aminayi, P. Magnetic properties of cobalt ferrite synthesized by hydrothermal method. Int. Nano Lett. 2015, 5, 183–186. [Google Scholar] [CrossRef] [Green Version]
- Cai, B.; Zhao, M.; Ma, Y.; Ye, Z.; Huang, J. Bioinspired formation of 3D hierarchical CoFe2O4 porous microspheres for magnetic-controlled drug release. ACS Appl. Mater. Interfaces 2015, 7, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, C.; Wu, J.; Ma, X.; Yang, D. Cobalt ferrite nanorings: Ostwald ripening dictated synthesis and magnetic properties. Chem. Commun. 2008, 43, 5648–5650. [Google Scholar] [CrossRef]
- Jovanović, S.; Spreitzer, M.; Tramšek, M.; Trontelj, Z.; Suvorov, D. Effect of oleic acid concentration on the physicochemical properties of cobalt ferrite nanoparticles. J. Phys. Chem. C 2014, 118, 13844–13856. [Google Scholar] [CrossRef]
- Repko, A.; Nižňanský, D.; Poltierová-Vejpravová, J. A study of oleic acid-based hydrothermal preparation of CoFe2O4 nanoparticles. J. Nanoparticle Res. 2011, 13, 5021–5031. [Google Scholar] [CrossRef]
- Rajput, A.B.; Hazra, S.; Ghosh, N.N. Synthesis and characterisation of pure single-phase CoFe2O4 nanopowder via a simple aqueous solution-based EDTA-precursor route. J. Exp. Nanosci. 2013, 8, 629–639. [Google Scholar] [CrossRef]
- Chandramohan, P.; Srinivasan, M.P.; Velmurugan, S.; Narasimhan, V.S. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 2011, 184, 89–96. [Google Scholar] [CrossRef]
- Nakagomi, F.; da Silva, S.W.; Garg, V.K.; Oliveira, A.C.; Morais, P.C.; Franco, A. Influence of the Mg-content on the cation distribution in cubic MgxFe3-xO4 nanoparticles. J. Solid State Chem. 2009, 182, 2423–2429. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, C.; Wu, K.; Zhou, H.; Hu, Y.; Preis, S. Mechanistic evaluation of ferrite AFe2O4 (A = Co, Ni, Cu, and Zn) catalytic performance in oxalic acid ozonation. Appl. Catal. A Gen. 2017, 547, 60–68. [Google Scholar] [CrossRef]
- Dippong, T.; Cadar, O.; Levei, E.A.; Bibicu, I.; Diamandescu, L.; Leostean, C.; Lazar, M.; Borodi, G.; Tudoran, L.B. Structure and magnetic properties of CoFe2O4/SiO2 nanocomposites obtained by sol-gel and post annealing pathways. Ceram. Int. 2017, 43, 2113–2122. [Google Scholar] [CrossRef]
- Stoia, M.; Stefanescu, M.; Dippong, T.; Stefanescu, O.; Barvinschi, P. Low temperature synthesis of Co2SiO4/SiO2 nanocomposite using a modified sol-gel method. J. Sol-Gel Sci. Technol. 2010, 54, 49–56. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Shafi, K.V.P.M.; Gedanken, A.; Prozorov, R.; Balogh, J. Sonochemical preparation and size-dependent properties of nanostructured CoFe2O4 particles. Chem. Mater. 1998, 10, 3445–3450. [Google Scholar] [CrossRef]
- Mohallem, N.D.S.; Seara, L.M. Magnetic nanocomposite thin films of NiFe2O4/SiO2 prepared by sol-gel process. Appl. Surf. Sci. 2003, 214, 143–150. [Google Scholar] [CrossRef]
- Vázquez-Vázquez, C.; Lovelle, M.; Mateo, C.; López-Quintela, M.A.; Buján-Núñez, M.C.; Serantes, D.; Baldomir, D.; Rivas, J. Maqnetocaloric effect and size-dependent study of the magnetic properties of cobalt ferrite nanoparticles prepared by solvothermal synthesis. Phys. Status Solidi Appl. Mater. Sci. 2008, 205, 1358–1362. [Google Scholar] [CrossRef]
- Desautels, R.D.; Cadogan, J.M.; van Lierop, J. Spin dynamics in CoFe2O4 nanoparticles. J. Appl. Phys. 2009, 105, 103–106. [Google Scholar] [CrossRef]
- Yahya, M.; Hosni, F.; Hamzaoui, A.H. Synthesis and ESR study of transition from ferromagnetism to superparamagnetism in La0.8Sr0.2MnO3 nanomanganite. J. Intechopen 2019, I, 1–12. [Google Scholar]
- Zhang, L.; He, R.; Gu, H. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surface Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Girgis, E.; Wahsh, M.M.; Othman, A.G.; Bandhu, L.; Rao, K. Synthesis, magnetic and optical properties of core/shell Co1xZnxFe2O4/SiO2 nanoparticles. Nanoscale Res. Lett. 2011, 324, 2397–2403. [Google Scholar]
- Zubair, A.; Ahmad, Z.; Mahmood, A.; Cheong, W.; Ali, I.; Khan, M.A.; Chughtai, A.H.; Ashiq, M.N. Structural, morphological and magnetic properties of Eu-doped CoFe2O4 nano-ferrites. Results Phys. 2017, 7, 3203–3208. [Google Scholar] [CrossRef]
- Suharyadi, E.; Muzakki, A.; Nofrianti, A.; Istiqomah, N.I.; Kato, T.; Iwata, S. Photocatalytic activity of magnetic core-shell CoFe2O4@ZnO nanoparticles for purification of methylene blue. ACS Appl. Mater. Interfaces 2020, 10, 1–6. [Google Scholar] [CrossRef]















| Element | Weight (%) | Atomic (%) |
|---|---|---|
| O | 37.93 | 68.46 |
| Fe | 41.3 | 21.36 |
| Co | 20.77 | 10.18 |
| Total | 100 | 100 |
| Peak Number | Wavelength (cm−1) | Functional Group |
|---|---|---|
| 1 | 3368.55 | O–H |
| 2 | 2919.52 | CH2– |
| 3 | 2850 | CH2– |
| 4 | 1530.4 | COO– |
| 5 | 1406.1 | COO– |
| 6 | 585.2 | Fe–O |
| Samples | Ms (emu/g) | Hc (Oe) |
|---|---|---|
| 0 M | 54.08 | 4012.8 |
| 0.05 M | 47.53 | 841.9 |
| 0.1 M | 29 | 0 |
| 0.15 M | 32 | 0 |
| 1 M | 49 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, H.D.T.; Nguyen, D.T.; Kim, K.-S. Effects of Process Variables on Properties of CoFe2O4 Nanoparticles Prepared by Solvothermal Process. Nanomaterials 2021, 11, 3056. https://doi.org/10.3390/nano11113056
Duong HDT, Nguyen DT, Kim K-S. Effects of Process Variables on Properties of CoFe2O4 Nanoparticles Prepared by Solvothermal Process. Nanomaterials. 2021; 11(11):3056. https://doi.org/10.3390/nano11113056
Chicago/Turabian StyleDuong, Hong Diu Thi, Dung The Nguyen, and Kyo-Seon Kim. 2021. "Effects of Process Variables on Properties of CoFe2O4 Nanoparticles Prepared by Solvothermal Process" Nanomaterials 11, no. 11: 3056. https://doi.org/10.3390/nano11113056