Tuning Dielectric Loss of SiO2@CNTs for Electromagnetic Wave Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations and Electromagnetic Parameter Measurement
2.3. Methods
2.3.1. Synthesis of the SiO2
2.3.2. Synthesis of the SiO2@Fe(OH)x
2.3.3. Synthesis of the SiO2@Fe3C/Fe@NCNT
2.3.4. Synthesis of the SiO2@Fe(OH)x-GT
2.3.5. Synthesis of the SiO2@Fe3C/Fe@NCNT-GT
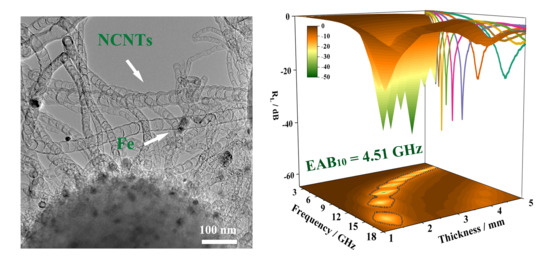
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, M.; Wang, X.; Zhang, M.; Cao, W.; Fang, X.; Yuan, J. Variable-Temperature Electron Transport and Dipole Polarization Turning Flexible Multifunctional Microsensor beyond Electrical and Optical Energy. Adv. Mater. 2020, 32, e1907156. [Google Scholar] [CrossRef] [PubMed]
- Yakovenko, O.; Matzui, L.; Vovchenko, L.; Lozitsky, O.; Prokopov, O.; Lazarenko, O.; Zhuravkov, A.; Oliynyk, V.; Launets, V.; Trukhanov, S. Electrophysical properties of epoxy-based composites with graphite nanoplatelets and magnetically aligned magnetite. Mol. Cryst. Liq. Cryst. 2018, 661, 68–80. [Google Scholar] [CrossRef]
- Yakovenko, O.S.; Matzui, L.Y.; Vovchenko, L.L.; Oliynyk, V.V.; Trukhanov, A.V.; Trukhanov, S.V.; Borovoy, M.O.; Tesel’ko, P.O.; Launets, V.L.; Syvolozhskyi, O.A.; et al. Effect of magnetic fillers and their orientation on the electrodynamic properties of BaFe12–xGaxO19 (x = 0.1–1.2)—epoxy composites with carbon nanotubes within GHz range. Appl. Nanosci. 2020, 10, 4747–4752. [Google Scholar] [CrossRef]
- Arief, I.; Biswas, S.; Bose, S. FeCo-anchored reduced graphene oxide framework-based soft composites containing carbon nanotubes as highly efficient microwave absorbers with excellent heat dissipation ability. ACS Appl. Mater. Interfaces 2017, 9, 19202–19214. [Google Scholar] [CrossRef] [PubMed]
- Vinnik, D.A.; Zhivulin, V.E.; Sherstyuk, D.P.; Starikov, A.Y.; Zezyulina, P.A.; Gudkova, S.A.; Zherebtsov, D.A.; Rozanov, K.N.; Trukhanov, S.V.; Astapovich, K.A.; et al. Electromagnetic properties of zinc–nickel ferrites in the frequency range of 0.05–10 GHz. Mater. Today Chem. 2021, 20, 100460. [Google Scholar] [CrossRef]
- Zdorovets, M.V.; Kozlovskiy, A.L.; Shlimas, D.I.; Borgekov, D.B. Phase transformations in FeCo–Fe2CoO4/Co3O4-spinel nanostructures as a result of thermal annealing and their practical application. J. Mater. Sci. Mater. Electron. 2021, 32, 16694–16705. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, N.; Bhatti, M.; Sharma, M.; Trukhanov, A.V.; Trukhanov, S.V.; Panina, L.V.; Astapovich, K.A.; Thakur, P. Synthesis of barium ferrite nano-particles using rhizome extract of Acorus Calamus: Characterization and its efficacy against different plant phytopathogenic fungi. Nano-Struct. Nano-Objects 2020, 24, 100599. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zdorovets, M. Effect of doping of Ce4+/3+ on optical, strength and shielding properties of (0.5−x)TeO2-0.25MoO-0.25Bi2O3-xCeO2 glasses. Mater. Chem. Phys. 2021, 263, 124444. [Google Scholar] [CrossRef]
- Wu, Z.; Pei, K.; Xing, L.; Yu, X.; You, W.; Che, R. Enhanced Microwave Absorption Performance from Magnetic Coupling of Magnetic Nanoparticles Suspended within Hierarchically Tubular Composite. Adv. Funct. Mater. 2019, 29, 1901448. [Google Scholar] [CrossRef]
- Lü, Y.; Wang, Y.; Li, H.; Lin, Y.; Jiang, Z.; Xie, Z.; Kuang, Q.; Zheng, L. MOF-Derived Porous Co/C Nanocomposites with Excellent Electromagnetic Wave Absorption Properties. ACS Appl. Mater. Interfaces 2015, 7, 13604–13611. [Google Scholar] [CrossRef]
- Yang, R.B.; Reddy, P.M.; Chang, C.J.; Chen, P.A.; Chen, J.K.; Chang, C.C. Synthesis and characterization of Fe3O4/polypyrrole/carbon nanotube composites with tunable microwave absorption properties: Role of carbon nanotube and polypyrrole content. Chem. Eng. J. 2016, 285, 497–507. [Google Scholar] [CrossRef]
- Li, F.; Zhan, W.; Su, Y.; Siyal, S.H.; Bai, G.; Xiao, W.; Zhou, A.; Sui, G.; Yang, X. Achieving excellent electromagnetic wave absorption of ZnFe2O4@CNT/polyvinylidene fluoride flexible composite membranes by adjusting processing conditions. Compos. Part A: Appl. Sci. Manuf. 2020, 133, 105866. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, X.; Lu, J.; Liu, J.; Wang, G.; Gong, H. Enhanced electromagnetic wave absorption performance of SiCN(Fe) fibers by in-situ generated Fe3Si and CNTs. Ceram. Int. 2021, 47, 19582–19594. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, X.; Chen, M.; Yu, R. Controllable permittivity in 3D Fe3O4/CNTs network for remarkable microwave absorption performances. RSC Adv. 2017, 7, 26801–26808. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, X.; Yuan, H.; Zhang, S.; Zhu, C.; Zhang, X.; Chen, Y. N-doped reduced graphene oxide aerogels containing pod-like N-doped carbon nanotubes and FeNi nanoparticles for electromagnetic wave absorption. Carbon 2020, 159, 357–365. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Yuan, H.; Zhang, S.; Ouyang, Q.; Zhu, C.; Zhang, X.; Chen, Y. Large-Scale Synthesis of Three-Dimensional Reduced Graphene Oxide/Nitrogen-Doped Carbon Nanotube Heteronanostructures as Highly Efficient Electromagnetic Wave Absorbing Materials. ACS Appl. Mater. Interfaces 2019, 11, 39100–39108. [Google Scholar] [CrossRef]
- Shu, R.; Li, W.; Zhou, X.; Tian, D.; Zhang, G.; Gan, Y.; Shi, J.-J.; He, J. Facile preparation and microwave absorption properties of RGO/MWCNTs/ZnFe2O4 hybrid nanocomposites. J. Alloy. Compd. 2018, 743, 163–174. [Google Scholar] [CrossRef]
- Yoshiyuki, N.; Kunihiro, S. Application of ferrite to electromagnetic wave absorber and its characteristics. IEEE Trans. Microw Theory Tech. 1971, 19, 65–72. [Google Scholar]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Cao, F.; Yan, F.; Xu, J.; Zhu, C.; Qi, L.; Li, C.; Chen, Y. Tailing size and impedance matching characteristic of nitrogen-doped carbon nanotubes for electromagnetic wave absorption. Carbon 2020, 174, 79–89. [Google Scholar] [CrossRef]
- Wei, H.; Yin, X.; Li, X.; Li, M.; Dang, X.; Zhang, L.; Cheng, L. Controllable synthesis of defective carbon nanotubes/Sc2Si2O7 ceramic with adjustable dielectric properties for broadband high-performance microwave absorption. Carbon 2019, 147, 276–283. [Google Scholar] [CrossRef]
- Liu, L.; Yan, F.; Li, K.; Zhu, C.; Xie, Y.; Zhang, X.; Chen, Y. Ultrasmall FeNi3N particles with an exposed active (110) surface an-chored on nitrogen-doped graphene for multifunctional electrocatalysts. J. Mater. Chem. A. 2019, 7, 1083–1091. [Google Scholar] [CrossRef]
- Wu, H.; Yang, T.; Du, Y.; Shen, L.; Ho, G.W. Identification of Facet-Governing Reactivity in Hematite for Oxygen Evolution. Adv. Mater. 2018, 30, e1804341. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Trukhanov, A.V.; Baykal, A.; Gungunes, H.; Trukhanova, E.L.; Kostishin, V.G. Strong correlation between Dy3+ concentration, structure, magnetic and microwave properties of the [Ni0.5Co0.5](DyxFe2−x)O4 nanosized ferrites. J. Ind. Eng. Chem. 2020, 90, 251–259. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Egizbek, K.; Zdorovets, M.V.; Ibragimova, M.; Shumskaya, A.; Rogachev, A.A.; Ignatovich, Z.V.; Kadyrzhanov, K. Evaluation of the effi-ciency of detection and capture of manganese in aqueous solutions of FeCeOx nanocomposites doped with Nb2O5. Sensors. 2020, 20, 4851. [Google Scholar] [CrossRef] [PubMed]
- Quan, B.; Gu, W.; Sheng, J.; Lv, X.; Mao, Y.; Liu, L.; Huang, X.; Tian, Z.; Ji, G. From intrinsic dielectric loss to geometry patterns: Dual-principles strategy for ultrabroad band microwave absorption. Nano Res. 2020, 14, 1495–1501. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, J.; Gu, W.; Zhao, H.; Zheng, J.; Zhang, B.; Ji, G. Cellulose-chitosan framework/polyailine hybrid aerogel toward thermal insulation and microwave absorbing application. Chem. Eng. J. 2020, 395, 125190. [Google Scholar] [CrossRef]
- Dolmatov, A.; Maklakov, S.; Zezyulina, P.; Osipov, A.; Petrov, D.; Naboko, A.; Polozov, V.; Maklakov, S.; Starostenko, S.; Lagarkov, A. Deposition of a SiO2 Shell of Variable Thickness and Chemical Composition to Carbonyl Iron: Synthesis and Microwave Measurements. Sensors 2021, 21, 4624. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Deshmukh, K.; Ahamed, M.; Pasha, S.K. Recent advances in electromagnetic interference shielding properties of metal and carbon filler reinforced flexible polymer composites: A review. Compos. Part A: Appl. Sci. Manuf. 2018, 114, 49–71. [Google Scholar] [CrossRef]
- Jacobo, S.E.; Aphesteguy, J.C.; Anton, R.L.; Schegoleva, N.; Kurlyandskaya, G. Influence of the preparation procedure on the properties of polyaniline based magnetic composites. Eur. Polym. J. 2007, 43, 1333–1346. [Google Scholar] [CrossRef]
- Liu, J.; Che, R.; Chen, H.; Zhang, F.; Xia, F.; Wu, Q.; Wang, M. Microwave Absorption Enhancement of Multifunctional Composite Microspheres with Spinel Fe3O4 Cores and Anatase TiO2 Shells. Small 2012, 8, 1214–1221. [Google Scholar] [CrossRef]
- Cao, M.; Song, W.; Hou, Z.; Wen, B.; Yuan, J. The effects of temperature and frequency on the dielectric properties, electro-magnetic interference shielding and microwave-absorption of short carbon fiber/silica composites. Carbon 2010, 48, 788–796. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Zhang, G.; Huang, Y.; You, W.; Che, R. Hollow Engineering to Co@N-Doped Carbon Nanocages via Synergistic Protecting-Etching Strategy for Ultrahigh Microwave Absorption. Adv. Funct. Mater. 2021, 31, 2102812. [Google Scholar] [CrossRef]
- Gu, W.; Cui, X.; Zheng, J.; Yu, J.; Zhao, Y.; Ji, G. Heterostructure design of Fe3N alloy/porous carbon nanosheet composites for efficient microwave attenuation. J. Mater. Sci. Technol. 2020, 67, 265–272. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, Q.; Bi, H.; Liang, C.; Yuan, K.; She, W.; Yang, Y.; Che, R. CoNi@SiO2@TiO2 and CoNi@Air@TiO2 microspheres with strong wideband microwave absorption. Adv. Mate. 2016, 28, 486–490. [Google Scholar] [CrossRef]
- Feng, J.; Pu, F.; Li, Z.; Li, X.; Hu, X.; Bai, J. Interfacial interactions and synergistic effect of CoNi nanocrystals and nitrogen-doped graphene in a composite microwave absorber. Carbon 2016, 104, 214–225. [Google Scholar] [CrossRef]
- Zhao, T.; Ji, X.; Jin, W.; Wang, C.; Ma, W.; Gao, J.; Dang, A.; Li, T.; Shang, S.; Zhou, Z. Direct in situ synthesis of a 3D interlinked amorphous carbon nanotube/graphene/BaFe12O19 composite and its electromagnetic wave absorbing properties. RSC Adv. 2017, 7, 15903–15910. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xiang, L.; Wei, W.; An, J.; He, J.; Gong, C.; Hou, Y. Efficient and Lightweight Electromagnetic Wave Absorber Derived from Metal Organic Framework-Encapsulated Cobalt Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 42102–42110. [Google Scholar] [CrossRef]
- Zhang, X.F.; Dong, X.L.; Huang, H.; Liu, Y.Y.; Wang, W.N.; Zhu, X.G.; Lv, B.; Lei, J.P. Microwave absorption properties of the car-bon-coated nickel nanocapsules. Appl Phys. Lett. 2006, 89, 053115. [Google Scholar] [CrossRef]
- Liu, X.; Cui, X.; Chen, Y.; Zhang, X.-J.; Yu, R.; Wang, G.-S.; Ma, H. Modulation of electromagnetic wave absorption by carbon shell thickness in carbon encapsulated magnetite nanospindles–poly(vinylidene fluoride) composites. Carbon 2015, 95, 870–878. [Google Scholar] [CrossRef]
- Mo, Z.C.; Yang, R.L.; Lu, D.W.; Yang, L.L.; Hu, Q.M.; Li, H.B.; Zhu, H.; Tang, Z.; Gui, X. Lightweight, three-dimensional carbon nanotube@TiO2 sponge with enhanced microwave absorption performance. Carbon 2019, 144, 433–439. [Google Scholar] [CrossRef]
- Wen, F.; Zhang, F.; Liu, Z. Investigation on Microwave Absorption Properties for Multiwalled Carbon Nanotubes/Fe/Co/Ni Nanopowders as Lightweight Absorbers. J. Phys. Chem. C 2011, 115, 14025–14030. [Google Scholar] [CrossRef]
- Zhou, Y.; Miao, J.; Shen, Y.; Xie, A. Novel porous FexCyNz/N-doped CNT nanocomposites with excellent bifunctions for cat-alyzing oxygen reduction reaction and absorbing electromagnetic wave. Appl. Surf. Sci. 2018, 453, 83–92. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Li, Y.; Yang, F.; Zhang, L.; Liu, L.; Ren, X.; Yang, H. Synthesis and microwave absorption property of flexible magnetic film based on graphene oxide/carbon nanotubes and Fe3O4 nanoparticles. J. Mater. Chem. A 2014, 2, 14940–14946. [Google Scholar] [CrossRef]
- Qi, X.; Xu, J.; Hu, Q.; Deng, Y.; Xie, R.; Jiang, Y.; Zhong, W.; Du, Y. Metal-free carbon nanotubes: Synthesis, and enhanced intrinsic mi-crowave absorption properties. Sci. Rep. 2016, 6, 28310. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Zhu, H.; Guo, H.; Yu, L. Investigation of the microwave-absorbing properties of Fe-filled carbon nanotubes. Mater. Lett. 2007, 61, 3547–3550. [Google Scholar] [CrossRef]
- Zhang, T.; Zhong, B.; Yang, J.Q.; Huang, X.X.; Wen, G. Boron and nitrogen doped carbon nanotubes/Fe3O4 composite archi-tectures with microwave absorption property. Ceram. Int. 2015, 41, 8163–8170. [Google Scholar] [CrossRef]
- Zou, T.; Li, H.; Zhao, N.; Shi, C. Electromagnetic and microwave absorbing properties of multi-walled carbon nanotubes filled with Ni nanowire. J. Alloy. Compd. 2010, 496, L22–L24. [Google Scholar] [CrossRef]
- Xu, P.; Han, X.J.; Liu, X.R.; Zhang, B.; Wang, C.; Wang, X.H. A study of the magnetic and electromagnetic properties of γ-Fe2O3–multiwalled carbon nanotubes (MWCNT) and Fe/Fe3C–MWCNT composites. Mater. Chem. Phys. 2009, 114, 556–560. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, H.; Song, Y.; Zhang, Y.; Huang, Y. The electromagnetic characteristics and absorbing properties of multi-walled carbon nanotubes filled with Er2O3 nanoparticles as microwave absorbers. Mater. Sci. Eng. B 2008, 153, 78–82. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, H. Dielectric, magnetic, and microwave absorbing properties of multi-walled carbon nanotubes filled with Sm2O3 nanoparticles. Mater. Lett. 2009, 63, 272–274. [Google Scholar] [CrossRef]
- Green, M.; Van Tran, A.T.; Chen, X. Maximizing the microwave absorption performance of polypyrrole by data-driven discovery. Compos. Sci. Technol. 2020, 199, 108332. [Google Scholar] [CrossRef]
- Green, M.; Tran, A.T.; Chen, X. Obtaining strong, broadband microwave absorption of polyaniline through data-driven ma-terials discovery. Adv. Mater. Interfaces 2020, 7, 2000658. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, F.; Xu, J.; Zhang, X.; Li, B.; Zhang, X.; Ouyang, Q.; Zhang, X.; Chen, Y. Tuning Dielectric Loss of SiO2@CNTs for Electromagnetic Wave Absorption. Nanomaterials 2021, 11, 2636. https://doi.org/10.3390/nano11102636
Cao F, Xu J, Zhang X, Li B, Zhang X, Ouyang Q, Zhang X, Chen Y. Tuning Dielectric Loss of SiO2@CNTs for Electromagnetic Wave Absorption. Nanomaterials. 2021; 11(10):2636. https://doi.org/10.3390/nano11102636
Chicago/Turabian StyleCao, Fenghui, Jia Xu, Xinci Zhang, Bei Li, Xiao Zhang, Qiuyun Ouyang, Xitian Zhang, and Yujin Chen. 2021. "Tuning Dielectric Loss of SiO2@CNTs for Electromagnetic Wave Absorption" Nanomaterials 11, no. 10: 2636. https://doi.org/10.3390/nano11102636