Polyethylene Glycol as Shape and Size Controller for the Hydrothermal Synthesis of SrTiO3 Cubes and Polyhedra
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. SrTiO3 Materials Synthesis
2.3. Materials Characterization
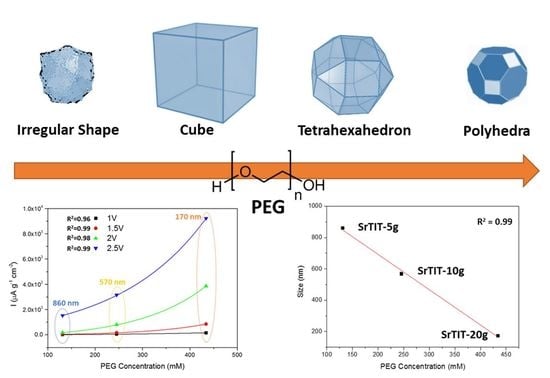
3. Results and Discussion
3.1. X-ray Diffraction
3.2. Shape and Size Characterization by Electron Microscopy and Dynamic Light Scattering
3.3. UV–Vis–NIR Spectroscopy
3.4. Photoelectrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Canu, G.; Buscaglia, V. Hydrothermal synthesis of strontium titanate: Thermodynamic considerations, morphology control and crystallisation mechanisms. CrystEngComm 2017, 19, 3867–3891. [Google Scholar] [CrossRef]
- Deak, D.S. Strontium titanate surfaces. Mater. Sci. Technol. 2013, 23, 127–136. [Google Scholar] [CrossRef]
- Pellegrino, F.; Sordello, F.; Mino, L.; Minero, C.; Hodoroaba, V.-D.; Martra, G.; Maurino, V. Formic Acid Photoreforming for Hydrogen Production on Shape-Controlled Anatase TiO2 Nanoparticles: Assessment of the Role of Fluorides, {101}/{001} Surfaces Ratio, and Platinization. ACS Catal. 2019, 9, 6692–6697. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the Photonic Crystal Properties of TiO2 Nanotube Arrays To Enhance Photocatalytic Hydrogen Production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous synthesis of TiO2 nanocrystals using TiF4 to engineer morphology, oxygen vacancy concentration, and photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Calderone, V.R.; Testino, A.; Buscaglia, M.T.; Bassoli, M.; Bottino, C.; Viviani, M.; Buscaglia, V.; Nanni, P. Size and Shape Control of SrTiO3Particles Grown by Epitaxial Self-Assembly. Chem. Mater. 2006, 18, 1627–1633. [Google Scholar] [CrossRef]
- Pellegrino, F.; Morra, E.; Mino, L.; Martra, G.; Chiesa, M.; Maurino, V. Surface and Bulk Distribution of Fluorides and Ti3+ Species in TiO2 Nanosheets: Implications on Charge Carrier Dynamics and Photocatalysis. J. Phys. Chem. C 2020, 124, 3141–3149. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Wrighton, M.S.; Ellis, A.B.; Wolczanski, P.T.; Morse, D.L.; Abrahamson, H.B.; Ginley, D.S. Strontium titanate photoelectrodes. Efficient photoassisted electrolysis of water at zero applied potential. J. Am. Chem. Soc. 1976, 98, 2774–2779. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Juan, J.C.; Show, P.-L.; Pan, G.-T. Recent developments of strontium titanate for photocatalytic water splitting application. Int. J. Hydrogen Energy 2019, 44, 14316–14340. [Google Scholar] [CrossRef]
- Shoji, S.; Yamaguchi, A.; Sakai, E.; Miyauchi, M. Strontium Titanate Based Artificial Leaf Loaded with Reduction and Oxidation Cocatalysts for Selective CO2 Reduction Using Water as an Electron Donor. ACS Appl. Mater. Interfaces 2017, 9, 20613–20619. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.H.; Lyu, H.; Hisatomi, T.; Goto, Y.; Takata, T.; Katayama, M.; Minegishi, T.; Domen, K. Efficient Photocatalytic Water Splitting Using Al-Doped SrTiO3 Coloaded with Molybdenum Oxide and Rhodium–Chromium Oxide. ACS Catal. 2018, 8, 2782–2788. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Kuang, Q.; Yang, S. Template synthesis of single-crystal-like porous SrTiO(3) nanocube assemblies and their enhanced photocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2013, 5, 3683–3690. [Google Scholar] [CrossRef]
- Crosby, L.A.; Chen, B.-R.; Kennedy, R.M.; Wen, J.; Poeppelmeier, K.R.; Bedzyk, M.J.; Marks, L.D. All Roads Lead to TiO2: TiO2-Rich Surfaces of Barium and Strontium Titanate Prepared by Hydrothermal Synthesis. Chem. Mater. 2018, 30, 841–846. [Google Scholar] [CrossRef]
- Mao, Y.; Park, T.J.; Wong, S.S. Synthesis of classes of ternary metal oxide nanostructures. Chem. Commun. 2005. [Google Scholar] [CrossRef]
- Dang, F.; Mimura, K.-I.; Kato, K.; Imai, H.; Wada, S.; Haneda, H.; Kuwabara, M. Growth of monodispersed SrTiO3 nanocubes by thermohydrolysis method. CrystEngComm 2011, 13, 3878. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, X.; Li, L. Highly dispersed SrTiO(3) nanocubes from a rapid sol-precipitation method. Nanoscale 2014, 6, 7940–7946. [Google Scholar] [CrossRef]
- Crosby, L.A.; Kennedy, R.M.; Chen, B.R.; Wen, J.; Poeppelmeier, K.R.; Bedzyk, M.J.; Marks, L.D. Complex surface structure of (110) terminated strontium titanate nanododecahedra. Nanoscale 2016, 8, 16606–16611. [Google Scholar] [CrossRef]
- Joshi, U.A.; Lee, J.S. Template-free hydrothermal synthesis of single-crystalline barium titanate and strontium titanate nanowires. Small 2005, 1, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Din, U.K.N.; Aziz, T.H.T.; Salleh, M.M.; Umar, A.A. Synthesis of crystalline perovskite-structured SrTiO3 nanoparticles using an alkali hydrothermal process. Int. J. Miner. Metall. Mater. 2016, 23, 109–115. [Google Scholar] [CrossRef]
- Kimijima, T.; Kanie, K.; Nakaya, M.; Muramatsu, A. Solvothermal synthesis of SrTiO3 nanoparticles precisely controlled in surface crystal planes and their photocatalytic activity. Appl. Catal. B Environ. 2014, 144, 462–467. [Google Scholar] [CrossRef]
- Xu, G.; Tao, Z.; Zhao, Y.; Zhang, Y.; Ren, Z.; Shen, G.; Han, G.; Wei, X. Solvothermal synthesis, characterization and formation mechanism of single-crystalline SrTiO3 dense spheres with monoethanolamine as reaction medium solvent. CrystEngComm 2013, 15, 1439. [Google Scholar] [CrossRef]
- Rabuffetti, F.A.; Kim, H.-S.; Enterkin, J.A.; Wang, Y.; Lanier, C.H.; Marks, L.D.; Poeppelmeier, K.R.; Stair, P.C. Synthesis-Dependent First-Order Raman Scattering in SrTiO3Nanocubes at Room Temperature. Chem. Mater. 2008, 20, 5628–5635. [Google Scholar] [CrossRef]
- Dong, L.; Shi, H.; Cheng, K.; Wang, Q.; Weng, W.; Han, W. Shape-controlled growth of SrTiO3 polyhedral submicro/nanocrystals. Nano Res. 2014, 7, 1311–1318. [Google Scholar] [CrossRef]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Hodoroaba, V.D.; Motzkus, C.; Mace, T.; Vaslin-Reimann, S. Performance of high-resolution SEM/EDX systems equipped with transmission mode (TSEM) for imaging and measurement of size and size distribution of spherical nanoparticles. Microsc. Microanal. 2014, 20, 602–612. [Google Scholar] [CrossRef]
- Hodoroaba, V.D.; Rades, S.; Salge, T.; Mielke, J.; Ortel, E.; Schmidt, R. Characterisation of nanoparticles by means of high-resolution SEM/EDS in transmission mode. IOP Conf. Ser. Mater. Sci. Eng. 2016, 109. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Shen, S.; Guo, L. SrTiO3 single crystals enclosed with high-indexed {023} facets and {001} facets for photocatalytic hydrogen and oxygen evolution. Appl. Catal. B Environ. 2015, 166–167, 320–326. [Google Scholar] [CrossRef]
- Hsieh, P.-L.; Naresh, G.; Huang, Y.-S.; Tsao, C.-W.; Hsu, Y.-J.; Chen, L.-J.; Huang, M.H. Shape-Tunable SrTiO3 Crystals Revealing Facet-Dependent Optical and Photocatalytic Properties. J. Phys. Chem. C 2019, 123, 13664–13671. [Google Scholar] [CrossRef]
- Mino, L.; Pellegrino, F.; Rades, S.; Radnik, J.; Hodoroaba, V.D.; Spoto, G.; Maurino, V.; Martra, G. Beyond Shape Engineering of TiO2 Nanoparticles: Post-Synthesis Treatment Dependence of Surface Hydration, Hydroxylation, Lewis Acidity and Photocatalytic Activity of TiO2 Anatase Nanoparticles with Dominant {001} or {101} Facets. ACS Appl. Nano Mater. 2018, 1, 5355–5365. [Google Scholar] [CrossRef] [Green Version]
- van Benthem, K.; Elsasser, C.; French, R.H. Bulk electronic structure of SrTiO3: Experiment and theory. J. Appl. Phys. 2001, 90, 6156–6164. [Google Scholar] [CrossRef] [Green Version]
- Xing, G.J.; Zhao, L.X.; Sun, T.; Su, Y.G.; Wang, X.J. Hydrothermal derived nitrogen doped SrTiO3 for efficient visible light driven photocatalytic reduction of chromium(VI). SpringerPlus 2016, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.A.; Chen, Q.L.; Meng, Q.Q.; Lei, S.L.; Li, C.Q.; Li, X.Y.; Ma, J.B. One-Step Synthesis of a Nanosized Cubic Li2TiO3-Coated Br, C, and N Co-Doped Li4Ti5O12 Anode Material for Stable High-Rate Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 25804–25816. [Google Scholar] [CrossRef]
- Snavely, D.L.; Dubsky, J. Near-IR spectra of polyethylene, polyethylene glycol, and polyvinylethyl ether. J. Polym. Sci. Pol. Chem. 1996, 34, 2575–2579. [Google Scholar] [CrossRef]
- Takeuchi, M.; Martra, G.; Coluccia, S.; Anpo, M. Evaluation of the adsorption states of H2O on oxide surfaces by vibrational absorption: Near- and mid-infrared spectroscopy. J. Near Infrared Spectrosc. 2009, 17, 373–384. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ozaki, Y.; Czarnecki, M.A. Molecular structure and hydrogen bonding in pure liquid ethylene glycol and ethylene glycol-water mixtures studied using NIR spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 18694–18701. [Google Scholar] [CrossRef] [Green Version]
- Mino, L.; Spoto, G.; Bordiga, S.; Zecchina, A. Particles Morphology and Surface Properties As Investigated by HRTEM, FTIR, and Periodic DFT Calculations: From Pyrogenic TiO2 (P25) to Nanoanatase. J. Phys. Chem. C 2012, 116, 17008–17018. [Google Scholar] [CrossRef]
- Mino, L.; Spoto, G.; Bordiga, S.; Zecchina, A. Rutile Surface Properties Beyond the Single Crystal Approach: New Insights from the Experimental Investigation of Different Polycrystalline Samples and Periodic DFT Calculations. J. Phys. Chem. C 2013, 117, 11186–11196. [Google Scholar] [CrossRef]
- Dong, B.; Liu, T.; Li, C.; Zhang, F. Species, engineering and characterizations of defects in TiO2 -based photocatalyst. Chin. Chem. Lett. 2018, 29, 671–680. [Google Scholar] [CrossRef]
- Llansola-Portoles, M.J.; Bergkamp, J.J.; Finkelstein-Shapiro, D.; Sherman, B.D.; Kodis, G.; Dimitrijevic, N.M.; Gust, D.; Moore, T.A.; Moore, A.L. Controlling surface defects and photophysics in TiO2 nanoparticles. J. Phys. Chem. A 2014, 118, 10631–10638. [Google Scholar] [CrossRef] [PubMed]
- Fazio, G.; Ferrighi, L.; Di Valentin, C. Photoexcited carriers recombination and trapping in spherical vs faceted TiO2 nanoparticles. Nano Energy 2016, 27, 673–689. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.-D.; Maurino, V. Influence of agglomeration and aggregation on the photocatalytic activity of TiO2 nanoparticles. Appl. Catal. B Environ. 2017, 216, 80–87. [Google Scholar] [CrossRef]








| Material | [PEG], mM | DH, nm | Size TEM, nm | Crystallographic Phase | Shape |
|---|---|---|---|---|---|
| SrTIT-0 g | 0 | 239 ± 52 | 177 ± 56 | SrTiO3 | Cuboidal and undefined |
| SrTIT-5 g | 131 | 680 ± 43 | 861 ± 122 | SrTiO3 | Cubes |
| SrTIT-10 g | 245 | 417 ± 20 | 569 ± 118 | SrTiO3 | Tetra-hexahedron |
| SrTIT-20 g | 434 | 236 ± 17 | 173 ± 33 | SrTiO3 | Polyhedra |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, F.; Sordello, F.; Mino, L.; Prozzi, M.; Mansfeld, U.; Hodoroaba, V.-D.; Minero, C. Polyethylene Glycol as Shape and Size Controller for the Hydrothermal Synthesis of SrTiO3 Cubes and Polyhedra. Nanomaterials 2020, 10, 1892. https://doi.org/10.3390/nano10091892
Pellegrino F, Sordello F, Mino L, Prozzi M, Mansfeld U, Hodoroaba V-D, Minero C. Polyethylene Glycol as Shape and Size Controller for the Hydrothermal Synthesis of SrTiO3 Cubes and Polyhedra. Nanomaterials. 2020; 10(9):1892. https://doi.org/10.3390/nano10091892
Chicago/Turabian StylePellegrino, Francesco, Fabrizio Sordello, Lorenzo Mino, Marco Prozzi, Ulrich Mansfeld, Vasile-Dan Hodoroaba, and Claudio Minero. 2020. "Polyethylene Glycol as Shape and Size Controller for the Hydrothermal Synthesis of SrTiO3 Cubes and Polyhedra" Nanomaterials 10, no. 9: 1892. https://doi.org/10.3390/nano10091892