Carrier Transfer and Capture Kinetics of the TiO2/Ag2V4O11 Photocatalyst
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Fabrication of TiO2/Ag2V4O11
2.3. Characterizations
3. Results and Discussion
3.1. Characterization and Photocatalytic Performances
3.2. Characterizations of Energy Bands Structure
3.3. Characterization of the Carrier Kinetics
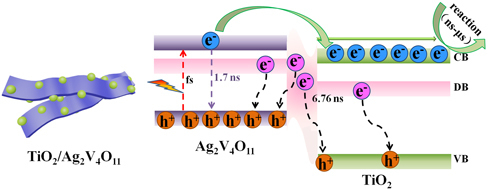
3.4. Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Ghosh, C.K.; Mukherjee, S.; Mukherjee, S.; Chattopadhyay, K.K. Three dimensional Ag2O/TiO2 Type-II (p-n) nanoheterojunctions for superior photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, B.; Yu, J. A new understanding of the photocatalytic mechanism of the direct Z-scheme g-C3N4/TiO2 heterostructure. Phys. Chem. Chem. Phys. 2016, 18, 31175–31183. [Google Scholar] [CrossRef]
- He, H.; Lin, J.; Fu, W.; Wang, X.; Wang, H.; Zeng, Q.; Gu, Q.; Li, Y.; Yan, C.; Tay, B.K.; et al. MoS2/TiO2 edge-On heterostructure for efficient photocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 1600464. [Google Scholar] [CrossRef]
- Jin, J.; Yu, J.; Guo, D.; Cui, C.; Ho, W. A hierarchical Z-Scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity. Small 2015, 11, 5262–5271. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, G.; Gao, T.; Zhang, H.; Yu, H. NiO nanosheet/TiO2 nanorod-constructed p–n heterostructures for improved photocatalytic activity. Appl. Surf. Sci. 2016, 364, 322–331. [Google Scholar] [CrossRef]
- Xu, Y.; Han, X.; Zheng, L.; Wei, S.; Xie, Y. First investigation on charge-discharge reaction mechanism of aqueous lithium ion batteries: A new anode material of Ag2V4O11 nanobelts. Dalton Trans. 2011, 40, 10751–10757. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yang, X.; Jiang, X.; Yu, A. Silver vanadate nanobelts: A highly sensitive material towards organic amines. Sens. Actuators B 2014, 203, 705–711. [Google Scholar] [CrossRef]
- Shi, H.F.; Li, Z.S.; Kou, J.H.; Ye, J.H.; Zou, Z.G. Facile synthesis of single-crystalline Ag2V4O11 nanotube material as a novel visible-light-sensitive photocatalyst. J. Phys. Chem. C 2011, 115, 145–151. [Google Scholar] [CrossRef]
- Biswas, S.; Husek, J.; Londo, S.; Baker, L.R. Highly localized charge transfer excitons in metal oxide semiconductors. Nano Lett. 2018, 18, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Schmuttenmaer, C.A. Exciton-like trap states limit electron mobility in TiO2 nanotubes. Nat. Nanotechnol. 2010, 5, 769–772. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.S.; Routh, P.K.; Zahl, P.; Nam, C.Y.; Cotlet, M. Distinct optoelectronic signatures for charge transfer and energy transfer in quantum dot–MoS2 hybrid photodetectors revealed by photocurrent imaging microscopy. Adv. Funct. Mater. 2018, 28, 1707558. [Google Scholar] [CrossRef]
- Redinger, A.; Levcenko, S.; Hages, C.J.; Greiner, D.; Kaufmann, C.A.; Unold, T. Time resolved photoluminescence on Cu(In, Ga)Se2 absorbers: Distinguishing degradation and trap states. Appl. Phys. Lett. 2017, 110, 122104. [Google Scholar] [CrossRef]
- Yamasa, T.; Yamada, Y.; Nishimura, H.; Nakaike, Y.; Wakamiya, A.; Murata, Y.; Kanemitsu, Y. Fast free-carrier diffusion in CH3NH3PbBr3 single crystals revealed by time-resolved one- and two-photon excitation photoluminescence spectroscopy. Adv. Electron. Mater. 2016, 2, 1500290. [Google Scholar]
- Wang, X.; Shen, S.; Feng, Z.; Li, C. Time-resolved photoluminescence of anatase/rutile TiO2 phase junction revealing charge separation dynamics. Chin. J. Catal. 2016, 37, 2059–2068. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Tian, J.; Wang, X.; Cui, H. TiO2 nanobelts decorated with In2S3 nanoparticles as photocatalysts with enhanced full-solar-spectrum (UV-vis-NIR) photocatalytic activity toward the degradation of tetracycline. Part. Part. Syst. Char. 2017, 34, 1700127. [Google Scholar] [CrossRef]
- Komarala, V.K.; Xie, C.; Wang, Y.; Xu, J.; Xiao, M. Time-resolved photoluminescence properties of CuInS2/ZnS nanocrystals: Influence of intrinsic defects and external impurities. J. Appl. Phys. 2012, 111, 124314. [Google Scholar] [CrossRef]
- Rocha, T.C.R.; Oestereich, A.; Demidov, D.V.; Hävecker, M.; Zafeiratos, S.; Weinberg, G.; Bukhtiyarov, V.I.; Knop-Gericke, A.; Schlögl, R. The silver–oxygen system in catalysis: New insights by near ambient pressure X-ray photoelectron spectroscopy. Phys. Chem. Chem. Phys. 2012, 14, 4554–4564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Tang, X.; Liu, J.; Zhan, E.; Li, J.; Huang, X.; Shen, W. Synthesis and characterization of Ag-hollandite nanofibers and its catalytic application in ethanol oxidation. Chem. Mater. 2007, 19, 4292–4299. [Google Scholar] [CrossRef]
- Zheng, J.; Calvillo, L.; Valero-Vidal, C.; Marga, C.; Sekar, P.; Shuang, S.; Girardi, L.; Agnoli, S.; Rizzi, G.; Granozzi, G. Ag-vanadates/GO nanocomposites by aerosol-assisted spray pyrolysis: Preparation and structural and electrochemical characterization of a versatile material. ACS Omega 2017, 2, 2792–2802. [Google Scholar] [CrossRef] [Green Version]
- Clavero, C. Plasmon induced hot-electron generation at nanoparticle metal oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Liang, F.; Yu, Y.; Zhou, W.; Xu, X.; Zhu, Z. Highly defective CeO2 as a promoter for efficient and stable water oxidation. J. Mater. Chem. A 2015, 3, 634–640. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Huang, Y.; Li, X.; Cao, X.; Xu, L.; Meng, C.; Wang, Z.; Zhu, W. Ag0.35V2O5/TiO2 branched nanoheterostructures: Facile fabrication and efficient visible light photocatalytic activity. Mater. Lett. 2014, 128, 358–361. [Google Scholar] [CrossRef]
- Hsiao, Y.; Wu, T.; Wang, Y.; Hu, C.; Huang, C. Evaluating the sensitizing effect on the photocatalytic decoloration of dyes using anatase-TiO2. Appl. Catal. B Environ. 2014, 148–149, 250–257. [Google Scholar] [CrossRef]
- Pan, L.; Zou, J.; Zhang, X.; Wang, L. Water-mediated promotion of dye sensitization of TiO2 under visible light. J. Am. Chem. Soc. 2011, 133, 10000–10002. [Google Scholar] [CrossRef]
- Molla, M.; Tateishi, I.; Tateishi, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Evaluation of reaction mechanism for photocatalytic degradation of dye with self-sensitized TiO2 under visible light irradiation. Open J. Inorg. Non-Met. Mater. 2017, 7, 1–7. [Google Scholar]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fujitsuka, M.; Du, Y.; Majima, T. 2D/2D heterostructured CdS/WS2 with efficient charge separation improving H2 evolution under visible light irradiation. ACS Appl. Mater. Interfaces 2018, 10, 20458–20466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Li, Z.; Zhang, L.; Pan, L.; Zhang, X.; Wang, L.; Aleem, F.; Zou, J.J. Role of oxygen vacancies in photocatalytic water oxidation on ceria oxide: Experiment and DFT studies. Appl. Catal. B Environ. 2018, 224, 101–108. [Google Scholar] [CrossRef]
- Wu, J.L.; Chen, F.C.; Hsiao, Y.S.; Chien, F.C.; Chen, P.L.; Kuo, C.H.; Huang, M.H.; Hsu, C.S. Surface plasmonic effects of metallic nanoparticles on the performance of polymer bulk heterojunction solar cells. ACS Nano 2011, 5, 959–967. [Google Scholar] [CrossRef]
- Elbanna, O.; Fujitsuka, M.; Majima, T. g-C3N4/TiO2 Mesocrystals Composite for H2 Evolution under Visible Light Irradiation and Its Charge Carriers Dynamics. ACS Appl. Mater. Interfaces 2017, 9, 34844–34854. [Google Scholar] [CrossRef]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Ding, Q.; Wang, Y.; OuYang, X.; Liu, L.; Li, J.; Wang, B. Carrier Transfer and Capture Kinetics of the TiO2/Ag2V4O11 Photocatalyst. Nanomaterials 2020, 10, 828. https://doi.org/10.3390/nano10050828
Zhou Y, Ding Q, Wang Y, OuYang X, Liu L, Li J, Wang B. Carrier Transfer and Capture Kinetics of the TiO2/Ag2V4O11 Photocatalyst. Nanomaterials. 2020; 10(5):828. https://doi.org/10.3390/nano10050828
Chicago/Turabian StyleZhou, Yun, Qiujie Ding, Yuan Wang, Xiaoping OuYang, Lixin Liu, Junyu Li, and Bing Wang. 2020. "Carrier Transfer and Capture Kinetics of the TiO2/Ag2V4O11 Photocatalyst" Nanomaterials 10, no. 5: 828. https://doi.org/10.3390/nano10050828