EMI Shielding of the Hydrophobic, Flexible, Lightweight Carbonless Nano-Plate Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Cu Nanoplate
2.3. Nonwoven Carbon Fabric Synthesis by Wet-Laid Method
2.4. Composite Preparation
2.5. Characterization
3. Results and Discussion
3.1. Scanning Electron Microscopic Analysis (SEM)
3.2. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.3. X-ray Diffraction Spectroscopy (XRD) Analysis
3.4. Raman Spectroscopy Analysis
3.5. Hydrophobic Property and Electric Conductivity
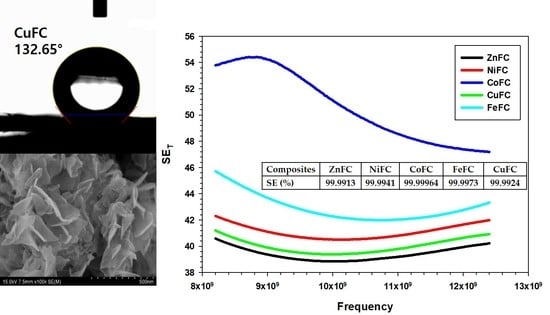
3.6. EMI Shielding of the Composites
3.6.1. EMI Shielding Theory and Mechanisms
3.6.2. EMI shielding of Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yun, T.; Kim, H.; Iqbal, A.; Cho, Y.S.; Lee, G.S.; Kim, M.; Kim, S.J.; Kim, D.; Gogotsi, Y.; Kim, S.O.; et al. Electromagnetic Shielding of Monolayer MXene Assemblies. Adv. Mater. 2020, 32, e1906769. [Google Scholar] [CrossRef] [PubMed]
- Rajavel, K.; Luo, S.; Wan, Y.; Yu, X.; Hu, Y.; Zhu, P.; Sun, R.; Wong, C. 2D Ti3C2Tx MXene/polyvinylidene fluoride (PVDF) nanocomposites for attenuation of electromagnetic radiation with excellent heat dissipation. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105693. [Google Scholar] [CrossRef]
- He, P.; Cao, M.-S.; Cai, Y.-Z.; Shu, J.-C.; Cao, W.-Q.; Yuan, J. Self-assembling flexible 2D carbide MXene film with tunable integrated electron migration and group relaxation toward energy storage and green EMI shielding. Carbon 2020, 157, 80–89. [Google Scholar] [CrossRef]
- Raagulan, K.; Braveenth, R.; Lee, L.R.; Lee, J.; Kim, B.M.; Moon, J.J.; Lee, S.B.; Chai, K.Y. Fabrication of Flexible, Lightweight, Magnetic Mushroom Gills and Coral-Like MXene⁻Carbon Nanotube Nanocomposites for EMI Shielding Application. Nanomaterials 2019, 9, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raagulan, K.; Braveenth, R.; Kim, B.M.; Lim, K.J.; Lee, S.B.; Kim, M.; Chai, K.Y. An effective utilization of MXene and its effect on electromagnetic interference shielding: Flexible, free-standing and thermally conductive composite from MXene–PAT–poly(p-aminophenol)–polyaniline co-polymer. RSC Adv. 2020, 10, 1613–1633. [Google Scholar] [CrossRef] [Green Version]
- Raagulan, K.; Kim, B.M.; Chai, K.Y. Recent Advancement of Electromagnetic Interference (EMI) Shielding of Two Dimensional (2D) MXene and Graphene Aerogel Composites. Nanomaterials. 2020, 10, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.-W.; Lee, S.-E.; Jeong, Y.G. Highly Effective Electromagnetic Interference Shielding Materials based on Silver Nanowire/Cellulose Papers. ACS Appl. Mater. Interfaces 2016, 8, 13123–13132. [Google Scholar] [CrossRef]
- Xie, H.; Zhou, Y.; Ren, Z.; Wei, X.; Tao, S.; Yang, C. Enhancement of electromagnetic interference shielding and heat-resistance properties of silver-coated carbonyl iron powders composite material. J. Magn. Magn. Mater. 2020, 499, 166244. [Google Scholar] [CrossRef]
- Xing, D.; Lu, L.-S.; Xie, Y.; Tang, Y.; Teh, K.S. Highly flexible and ultra-thin carbon-fabric/Ag/waterborne polyurethane film for ultra-efficient EMI shielding. Mater. Des. 2020, 185, 108227. [Google Scholar] [CrossRef]
- Zhu, D.; Ortega, C.F.; Motamedi, R.; Szewciw, L.; Vernerey, F.; Barthelat, F. Structure and Mechanical Performance of a “Modern” Fish Scale. Adv. Eng. Mater. 2011, 14, B185–B194. [Google Scholar] [CrossRef]
- Bian, R.; He, G.; Zhi, W.; Xiang, S.; Wang, T.; Cai, D. Ultralight MXene-based aerogels with high electromagnetic interference shielding performance. J. Mater. Chem. C 2019, 7, 474–478. [Google Scholar] [CrossRef]
- Ye, S.; Huang, H.; Yuan, C.; Liu, F.; Zhai, M.; Shi, X.; Qi, C.; Wang, G. Thickness-Dependent Strain Effect on the Deformation of the Graphene-Encapsulated Au Nanoparticles. J. Nanomater. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Raagulan, K.; Braveenth, R.; Jang, H.J.; Lee, Y.S.; Yang, C.-M.; Kim, B.M.; Moon, J.J.; Chai, K.Y. Electromagnetic Shielding by MXene-Graphene-PVDF Composite with Hydrophobic, Lightweight and Flexible Graphene Coated Fabric. Materials 2018, 11, 1803. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.; Ahmad, S.; Durrani, S.; Faiz, M.; Ul-Hamid, A. Application of nickel oxide thin films in NiO/Ag multilayer energy-efficient coatings. Mater. Sci. Semicond. Process. 2015, 39, 84–89. [Google Scholar] [CrossRef]
- Mullet, M.; Khare, V.; Ruby, C. XPS study of Fe (II) Fe (III)(oxy) hydroxycarbonate green rust compounds. Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces. Interfaces Thin Films 2008, 40, 323–328. [Google Scholar]
- Haider, M.B. XPS Depth Profile Analysis of Zn 3 N 2 Thin Films Grown at Different N 2/Ar Gas Flow Rates by RF Magnetron Sputtering. Nanoscale Res. Lett. 2017, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Mardiansyah, D.; Badloe, T.; Triyana, K.; Mehmood, M.Q.; Raeis-Hosseini, N.; Lee, Y.; Sabarman, H.; Kim, K.; Rho, J. Effect of temperature on the oxidation of Cu nanowires and development of an easy to produce, oxidation-resistant transparent conducting electrode using a PEDOT:PSS coating. Sci. Rep. 2018, 8, 10639. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K. Green synthesis of cobalt nanoparticles by using methanol extract of plant leaf as reducing agent. Pure Appl. Biol. 2016, 5, 453. [Google Scholar] [CrossRef]
- Lassoued, A.; Dkhil, B.; Gadri, A.; Ammar, S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Üzüm, Ç.; Shahwan, T.; Eroğlu, A.E.; Lieberwirth, I.; Scott, T.B.; Hallam, K.R. Application of zero-valent iron nanoparticles for the removal of aqueous Co2+ ions under various experimental conditions. Chem. Eng. J. 2008, 144, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, Y.; He, Y.; Swihart, M.T.; Wang, S.; Luo, H.; Furlani, E.P.; Prasad, P.N. An aerosol-mediated magnetic colloid: Study of nickel nanoparticles. J. Appl. Phys. 2005, 98, 054308. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.L.T.; Jee, M.S.; Won, D.H.; Jung, H.; Oh, H.-S.; Min, B.K.; Hwang, Y.J. Selective CO2 Reduction on Zinc Electrocatalyst: The Effect of Zinc Oxidation State Induced by Pretreatment Environment. ACS Sustain. Chem. Eng. 2017, 5, 11377–11386. [Google Scholar] [CrossRef]
- Gladunova, O.I.; Fedorova, Y.E.; Astashkina, O.V.; Lysenko, A.A. Composites with Hydrophobic Surfaces. Fibre Chem. 2015, 47, 317–319. [Google Scholar] [CrossRef]
- Vanslambrouck, S.; Chevallier, P.; Guay-Bégin, A.-A.; Laroche, G. Effect of linking arm hydrophilic/hydrophobic nature, length and end-group on the conformation and the RGD accessibility of surface-immobilized fibronectin. Mater. Sci. Eng. C 2020, 107, 110335. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Pathak, A.; Usman, M.; Chiou, K.-R.; Tsai, P.-H.; Mendiratta, S.; Kamal, S.; Liu, Y.-H.; Chen, J.; Chiang, M.-H.; et al. Highly hydrophobic metal–organic framework for self-protecting gate dielectrics. J. Mater. Chem. A 2020, 8, 11958–11965. [Google Scholar] [CrossRef]
- Han, M.; Shuck, C.E.; Rakhmanov, R.; Parchment, D.; Anasori, B.; Koo, C.M.; Friedman, G.; Gogotsi, Y. Beyond Ti3C2T x: MXenes for Electromagnetic Interference Shielding. ACS Nano 2020, 14, 5008–5016. [Google Scholar] [CrossRef]
- Chen, W.; Liu, L.-X.; Zhang, H.; Yu, Z. Flexible, Transparent, and Conductive Ti3C2Tx MXene–Silver Nanowire Films with Smart Acoustic Sensitivity for High-Performance Electromagnetic Interference Shielding. ACS Nano 2020. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, J.; Che, R.; Xu, J.; Liu, M.; Liu, Z. Double-Shelled Yolk–Shell Microspheres with Fe3O4 Cores and SnO2 Double Shells as High-Performance Microwave Absorbers. J. Phys. Chem. C 2012, 117, 489–495. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, W.; Shao, G.; Fan, B.; Zhang, R. Corrosive synthesis and enhanced electromagnetic absorption properties of hollow porous Ni/SnO2 hybrids. Dalton Trans. 2015, 44, 15984–15993. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Liang, M.; Yan, L.; Chen, Y.; Zou, H. Co/C@cellulose nanofiber aerogel derived from metal-organic frameworks for highly efficient electromagnetic interference shielding. Chem. Eng. J. 2020, 392, 124815. [Google Scholar] [CrossRef]
- Sushmita, K.; Madras, G.; Bose, S. Polymer Nanocomposites Containing Semiconductors as Advanced Materials for EMI Shielding. ACS Omega 2020, 5, 4705–4718. [Google Scholar] [CrossRef] [PubMed]
- Santhosi, B.; Ramji, K.; Rao, N.M. Design and development of polymeric nanocomposite reinforced with graphene for effective EMI shielding in X-band. Phys. B Condens. Matter 2020, 586, 412144. [Google Scholar] [CrossRef]
- Rajavel, K.; Hu, Y.; Zhu, P.; Sun, R.; Wong, C. MXene/metal oxides-Ag ternary nanostructures for electromagnetic interference shielding. Chem. Eng. J. 2020, 399, 125791. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Zhou, C.-G.; Sun, W.-J.; Wang, T.; Jia, L.-C.; Yan, D.-X.; Li, Z.-M. Injection molding of segregated carbon nanotube/polypropylene composite with enhanced electromagnetic interference shielding and mechanical performance. Compos. Sci. Technol. 2020, 197, 108253. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, N.; Yu, T.; Xi, J.; Mao, A.; Yuan, M.; Bai, H.; Gao, C. High-efficiency electromagnetic interference shielding realized in nacre-mimetic graphene/polymer composite with extremely low graphene loading. Carbon 2020, 157, 570–577. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, F.; Yue, Y.; Han, D.; Lin, L.; Zhao, S.; Zhao, Y.; Pan, Z.; Li, C.; Nyström, G.; et al. Flexible and Ultrathin Waterproof Cellular Membranes Based on High-Conjunction Metal-Wrapped Polymer Nanofibers for Electromagnetic Interference Shielding. Adv. Mater. 2020, 32, e1908496. [Google Scholar] [CrossRef]
- Bora, P.J.; Anil, A.G.; Ramamurthy, P.C.; Tan, D.Q. MXene interlayered crosslinked conducting polymer film for highly specific absorption and electromagnetic interference shielding. Mater. Adv. 2020, 1, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lin, S.; Huang, K.; Jia, C.; Wang, Q.; Li, Z.; Song, J.; Liu, Z.; Wang, H.; Lei, M.; et al. A large-area AgNW-modified textile with high-performance electromagnetic interference shielding. NPJ Flex. Electron. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, S.; Xing, C.; Pan, X.; Zuo, X.; Liu, J.; Chen, M.; Liu, L.; Tao, G.; Li, Q. Fe nanoparticles and CNTs co-decorated porous carbon/graphene foam composite for excellent electromagnetic interference shielding performance. J. Alloys Compd. 2020, 820, 153108. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.; Zhang, J.; Zhang, Y.; Gu, J. Lightweight and robust rGO/sugarcane derived hybrid carbon foams with outstanding EMI shielding performance. J. Mater. Sci. Technol. 2020, 52, 119–126. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, P.; Zhang, C.; Wang, Y.; Chang, H.; Rao, W. Porous and anisotropic liquid metal composites with tunable reflection ratio for low-temperature electromagnetic interference shielding. Appl. Mater. Today 2020, 19, 100612. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Gao, S.-P.; Jing, L.; Li, K.; Yang, H.; Fu, F.; Lee, J.Y.; Guo, Y.-X.; Ho, J.S.; et al. Reversible Crumpling of 2D Titanium Carbide (MXene) Nanocoatings for Stretchable Electromagnetic Shielding and Wearable Wireless Communication. Adv. Funct. Mater. 2019, 30, 1907451. [Google Scholar] [CrossRef]
- Park, S.I.; Kang, C.W.; Cho, S.Y.; Lee, S.M.; Kim, H.J.; Ko, Y.-J.; Choi, J.; Son, S.U. Fabrication of Poly(ethylene terephthalate) Fiber@Microporous Organic Polymer with Amino Groups@Cu Films for Flexible and Metal-Economical Electromagnetic Interference Shielding Materials. Langmuir 2020, 36, 8745–8752. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Tan, J.; Liu, J.; Zhu, M.; Zhu, B.; Zhang, Q. Bioinspired ultra-thin polyurethane/MXene nacre-like nanocomposite films with synergistic mechanical properties for electromagnetic interference shielding. J. Mater. Chem. C 2020, 8, 7170–7180. [Google Scholar] [CrossRef]
- Ma, Z.; Kang, S.; Ma, J.; Shao, L.; Zhang, Y.; Liu, C.; Wei, A.; Xiang, X.; Wei, L.; Gu, J. Ultraflexible and Mechanically Strong Double-Layered Aramid Nanofiber–Ti3C2Tx MXene/Silver Nanowire Nanocomposite Papers for High-Performance Electromagnetic Interference Shielding. ACS Nano 2020, 14, 8368–8382. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Zhang, H.; Chen, W.; Zhao, Z.; Wang, Q.; Yu, Z. Ultrastrong and Highly Conductive MXene-Based Films for High-Performance Electromagnetic Interference Shielding. Adv. Electron. Mater. 2019, 6, 1901094. [Google Scholar] [CrossRef]
- Gupta, S.; Chang, C.; Anbalagan, A.K.; Lee, C.-H.; Tai, N.-H. Reduced graphene oxide/zinc oxide coated wearable electrically conductive cotton textile for high microwave absorption. Compos. Sci. Technol. 2020, 188, 107994. [Google Scholar] [CrossRef]
- Weng, C.; Xing, T.; Jin, H.; Wang, G.; Dai, Z.; Pei, Y.; Kuang, J.; Zhang, Z. Mechanically robust ANF/MXene composite films with tunable electromagnetic interference shielding performance. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105927. [Google Scholar] [CrossRef]
- Lei, C.; Zhang, Y.; Liu, D.; Wu, K.; Fu, Q. Metal-Level Robust, Folding Endurance, and Highly Temperature-Stable MXene-Based Film with Engineered Aramid Nanofiber for Extreme-Condition Electromagnetic Interference Shielding Applications. ACS Appl. Mater. Interfaces 2020, 12, 26485–26495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Qi, Q.; Xu, N.; Yu, D. Layer-by-layer assembly of PDMS-coated nickel ferrite/multiwalled carbon nanotubes/cotton fabrics for robust and durable electromagnetic interference shielding. Cellular 2020, 27, 2829–2845. [Google Scholar] [CrossRef]
- Shukla, V. Role of spin disorder in magnetic and EMI shielding properties of Fe 3 O 4/C/PPy core/shell composites. J. Mater. Sci. 2020, 55, 2826–2835. [Google Scholar] [CrossRef]
- Wang, L.; Song, P.; Lin, C.-T.; Kong, J.; Gu, J. 3D Shapeable, Superior Electrically Conductive Cellulose Nanofibers/Ti3C2Tx MXene Aerogels/Epoxy Nanocomposites for Promising EMI Shielding. Research 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, J.; Peng, W.; Zhu, Y.; Zhao, Y.; Jiang, K.; Peng, M.; Tan, Y. Highly Stable 3D Ti3C2T x MXene-Based Foam Architectures toward High-Performance Terahertz Radiation Shielding. ACS Nano 2020, 14, 2109–2117. [Google Scholar] [CrossRef] [PubMed]













| Composite | C1s | O1s | F1s | N1s | S2p | Metal 2p |
|---|---|---|---|---|---|---|
| ZnFC | 79.90 | 11.32 | 2.11 | 2.75 | 3.08 | 0.84 |
| FeFC | 78.93 | 12.02 | 1.93 | 2.60 | 3.03 | 1.48 |
| NiFC | 68.74 | 12.36 | 12.18 | 2.00 | 2.26 | 2.00 |
| CoFC | 56.84 | 17.34 | 15.93 | 1.66 | 1.71 | 5.36 |
| CuFC | 82.23 | 10.37 | 1.16 | 2.81 | 3.05 | 0.39 |
| Composite | Contact Angle | Wetting Energy [mN/m] | Spreading Coefficient [mN/m] | Work of Adhesion [mN/m] |
|---|---|---|---|---|
| CoFC | 118.09 | −34.28 | −107.08 | 38.52 |
| CuFC | 132.65 | −49.32 | −122.12 | 23.48 |
| FeFC | 120.60 | −37.06 | −109.86 | 35.74 |
| NiFC | 66.90 | 28.56 | −44.24 | −101.36 |
| ZnFC | 116.60 | −32.60 | −105.40 | 40.20 |
| Parameters | CoFC | CuFC | FeFC | NiFC | ZnFC |
|---|---|---|---|---|---|
| Sheet resistance (ohm/sq) | 11.890 | 4.125 | 2.889 | 6.175 | 7.260 |
| Thickness (cm) | 0.0370 | 0.0282 | 0.0386 | 0.0330 | 0.0398 |
| resistivity (ρ=Rs.t) (Ω.cm) | 0.4399 | 0.1163 | 0.1115 | 0.2038 | 0.2889 |
| EC (σ = 1/(Rs.t)) (S.cm−1) | 2.2731 | 8.597 | 8.967 | 4.907 | 3.461 |
| Composites | ZnFC | NiFC | CoFC | FeFC | Cu15FC | Cu10FC | Cu5FC |
|---|---|---|---|---|---|---|---|
| MAX (dB) | 40.59 | 42.31 | 54.43 | 45.713 | 41.21 | 41.09 | 41.69 |
| Ave (dB) | 39.41 | 41.09 | 50.63 | 42.91 | 40.00 | 40.18 | 40.89 |
| Min (dB) | 38.84 | 40.51 | 47.19 | 41.98 | 39.39 | 39.99 | 40.63 |
| No. | Composite | Thickness (mm) | EC (S.cm−1) | SE (dB) | SE (%) | SE/d (dB.mm−1) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Metal-Wrapped Cellular Membranes | 0.005 | * | 76.8 | 99.9999979 | 15360 | [38] |
| 2 | PEDOT:PSS/Ti3C2Tx | 0.006 | * | 41 | 99.9921 | 6833.33 | [39] |
| 3 | AgNW-modified textile | 1.4 | * | 55 | 99.99968 | 39.29 | [40] |
| 4 | Fe nanoparticles and CNTs co-decorated porous carbon/graphene foam | 1 | * | 48 | 99.9984 | 48 | [41] |
| 5 | rGO/sugarcane | 3 | 6 | 53 | 99.99949 | 17.67 | [42] |
| 6 | liquid metal composites | 3 | * | 75 (77 K) 21.51 (300 K) | 99.9999968 | 25 | [43] |
| 7 | Titanium Carbide (MXene) Nanocoatings | 0.001 | 2900 | 52 | 99.99937 | 52000 | [44] |
| 8 | carbon-fabric/Ag/waterborne polyurethane | 0.183 | 11986.8 | 102.8 | 99.99999999 | 561.75 | [9] |
| 9 | PET Fiber@Microporous Organic Polymer with Amino Groups@Cu Films | 0.00064 | * | 73.8 | 99.9999958 | 114062.5 | [45] |
| 10 | polyurethane/MXene nacre-like nanocomposite films | 0.0074 | 5983.5 | 61.4 | 99.999928 | 8297.30 | [46] |
| 11 | Aramid Nanofiber–Ti3C2Tx MXene/Silver Nanowire Nanocomposite | 0.091 | 3725.6 | 79.8 | 99.9999989 | 876.92 | [47] |
| 12 | Conductive MXene-Based Films | 0.007 | 2638.83 | 50.17 | 99.99904 | 7244.29 | [48] |
| 13 | Reduced graphene oxide/zinc oxide coated conductive cotton textile | 0.00439 | 15.79 | 54.9 | 99.99968 | 12505.69 | [49] |
| 14 | ANF/MXene composite | 0.0032 | 879 | 40.6 | 99.9913 | 12687.5 | [50] |
| 15 | MXene- Aramid Nanofiber composite | 0.02137 | 36.618 | 34.6 | 99.9653 | 1616.10 | [51] |
| 16 | PDMS-coated nickel ferrite/MWCNT/cotton fabrics | 0.32 | 0.18 | 37 | 99.9800 | 115.63 | [52] |
| 17 | Fe3O4/C/PPy core/shell composites | 0.8 | * | 28 | 99.8415 | 35 | [53] |
| 18 | Cellulose Nanofibers/Ti3C2Tx MXene Aerogels/Epoxy Nanocomposites | 2 | 16.72 | 74 | 99.9999961 | 37 | [54] |
| 19 | MXene-poly(3,4ethylenedioxythiophene): poly(styrene sulfonate) (PEDOT:PSS) | 0.006 | * | 41 | 99.9921 | 7666.67 | [39] |
| 20 | Zn2+/Ti3C2Tx MXene-Based Foam | 0.085 | 56.718 | 51 | 99.99921 | 600 | [55] |
| 21 | ZnFC | 0.398 | 3.461 | 40.59 | 99.9913 | 101.99 | This work |
| 22 | NiFC | 0.330 | 4.907 | 42.31 | 99.9941 | 128.21 | |
| 23 | FeFC | 0.386 | 8.967 | 45.72 | 99.9973 | 110.67 | |
| 24 | CuFC | 0.282 | 8.597 | 41.21 | 99.9924 | 146.14 | |
| 25 | CoFC | 0.37 | 22.731 | 54.43 | 99.99964 | 147.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raagulan, K.; Ghim, J.S.; Braveenth, R.; Jung, M.J.; Lee, S.B.; Chai, K.Y.; Mi Kim, B.; Lee, J. EMI Shielding of the Hydrophobic, Flexible, Lightweight Carbonless Nano-Plate Composites. Nanomaterials 2020, 10, 2086. https://doi.org/10.3390/nano10102086
Raagulan K, Ghim JS, Braveenth R, Jung MJ, Lee SB, Chai KY, Mi Kim B, Lee J. EMI Shielding of the Hydrophobic, Flexible, Lightweight Carbonless Nano-Plate Composites. Nanomaterials. 2020; 10(10):2086. https://doi.org/10.3390/nano10102086
Chicago/Turabian StyleRaagulan, Kanthasamy, Jin Soo Ghim, Ramanaskanda Braveenth, Moon Jai Jung, Sang Bok Lee, Kyu Yun Chai, Bo Mi Kim, and Joonsik Lee. 2020. "EMI Shielding of the Hydrophobic, Flexible, Lightweight Carbonless Nano-Plate Composites" Nanomaterials 10, no. 10: 2086. https://doi.org/10.3390/nano10102086