Convenient and Controllable Synthesis of Poly(2-oxazoline)-Conjugated Doxorubicin for Regulating Anti-Tumor Selectivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Synthesis of N-Boc-2-bromoacetohydrazide
2.4. Polymerization of 2-Ethyl-oxazoline
2.5. Synthesis of Deprotected PEtOx
2.6. Conjugation of Doxorubicin and PEtOx
2.7. Purification and Characterization of DOX-PEtOx Conjugates
2.8. Self-Assembling Test of DOX-PEtOx Conjugate
2.9. In Vitro Cytotoxicity Assessment
3. Results and Discussion
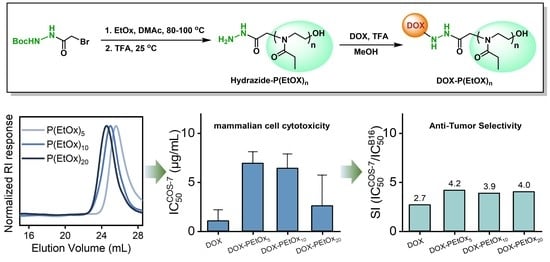
3.1. Synthesis and Characterization of Hydrazide End-Functional PEtOx
3.2. Synthesis and Characterization of DOX-PEtOx Conjugates
3.3. In Vitro Cytotoxicity and Anti-Tumor Efficacy of DOX-PEtOx Conjugates
3.4. Anti-Tumor Selectivity of DOX-PEtOx Conjugates
3.5. Self-Assembling Ability of DOX-PEtOx Conjugates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopecek, J. Polymer-drug Conjugates: Origins, Progress to Date and Future Directions. Adv. Drug Deliv. Rev. 2013, 65, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; He, J.; Zhang, M.; Ni, P. A pH-sensitive and Biodegradable Supramolecular Hydrogel Constructed from a PEGylated Polyphosphoester-doxorubicin Prodrug and α-cyclodextrin. Polym. Chem. 2015, 6, 5009–5014. [Google Scholar] [CrossRef]
- Zayed, G.M.; Kamal, I.; Abdelhafez, W.A.; Fahd, M.A.; Amin, M.A.; Shaykoon, M.S.A.; Sarhan, H.A.; Abdelsalam, A.M. Effect of Chemical Binding of Doxorubicin Hydrochloride to Gold Nanoparticles, Versus Electrostatic Adsorption, on the In Vitro Drug Release and Cytotoxicity to Breast Cancer Cells. J. Pharm. Sci. 2018, 35, 112. [Google Scholar] [CrossRef]
- Psarrou, M.; Kothri, M.G.; Vamvakaki, M. Photo- and Acid-Degradable Polyacylhydrazone-Doxorubicin Conjugates. Polymers 2021, 13, 2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, X.; Xu, B. PEGylated Doxorubicin Prodrug-Forming Reduction-Sensitive Micelles With High Drug Loading and Improved Anticancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 781982. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Shen, M.; Tomas, H.; Zhou, B.; Shi, X. Antitumor Efficacy of Doxorubicin-Loaded Electrospun Attapulgite-Poly(lactic-co-glycolic acid) Composite Nanofibers. J. Funct. Biomater. 2022, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hirai, Y.; Yuba, E.; Harada, A.; Kono, K. Temperature-Responsive Molecular Assemblies Using Oligo(Ethylene Glycol)-Attached Polyamidoamine Dendron Lipids and their Functions as Drug Carriers. J. Funct. Biomater. 2020, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, C.; Jia, R.; Gao, R.; Zhao, Y.; Ji, Q.; Cai, J.; Li, Q.; Wang, Y. PEG-poly(amino acid)s/EpCAM aptamer multifunctional nanoparticles arrest the growth and metastasis of colorectal cancer. Biomater. Sci. 2021, 9, 3705–3717. [Google Scholar] [CrossRef]
- Hoogenboom, R. The future of poly(2-oxazoline)s. Eur. Polym. J. 2022, 179, 111521. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, Q.; Zhang, W.; Liu, H.; Wan, J.; Qian, Y.; Li, B.; Tang, S.; Liu, Y.; Chen, S.; et al. Silk-Inspired β-Peptide Materials Resist Fouling and the Foreign-Body Response. Angew. Chem. Int. Ed. 2020, 59, 9586–9593. [Google Scholar] [CrossRef]
- Kagiya, T.; Narisawa, S.; Maeda, T.; Fukui, K. Ring-opening polymerization of 2-substituted 2-oxazolines. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 441–445. [Google Scholar] [CrossRef]
- Seeliger, W.; Aufderhaar, E.; Diepers, W.; Feinauer, R.; Nehring, R.; Thier, W.; Hellmann, H. Recent Syntheses and Reactions of Cyclic Imidic Esters. Angew. Chem. Int. Ed. 1966, 5, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Sheetz, D.P. Homopolymerization of 2-alkyl- and 2-aryl-2-oxazolines. J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 2253–2265. [Google Scholar] [CrossRef]
- Bassiri, T.G.; Levy, A.; Litt, M. Polymerization of cyclic imino ethers. I. Oxazolines. J. Polym. Sci. Part B Polym. Lett. 1967, 5, 871–879. [Google Scholar] [CrossRef]
- Dai, C.; Zhou, M.; Jiang, W.; Xiao, X.; Zou, J.; Qian, Y.; Cong, Z.; Ji, Z.; Liu, L.; Xie, J.; et al. Breaking or Following the Membrane-targeting Mechanism: Exploring the Antibacterial Mechanism of Host Defense Peptide Mimicking Poly(2-oxazoline)s. J. Mater. Sci. Technol. 2020, 59, 220–226. [Google Scholar] [CrossRef]
- Zhou, M.; Ji, Z.; Xiao, X.; Liu, L.; Cui, R.; Luo, Z.; Cong, Z.; Liu, R. Controllable and Facile Synthesis of Poly(2-oxazoline)s Using Trimethylsilyl Trifluoromethanesulfonate as the Initiator. Polym. Chem. 2023, 14, 1064–1071. [Google Scholar] [CrossRef]
- Zhou, M.; Jiang, W.; Xie, J.; Zhang, W.; Ji, Z.; Zou, J.; Cong, Z.; Xiao, X.; Gu, J.; Liu, R. Peptide-Mimicking Poly(2-oxazoline)s Displaying Potent Antimicrobial Properties. Chem. Med. Chem. 2021, 16, 309–315. [Google Scholar] [CrossRef]
- Zhou, M.; Qian, Y.; Xie, J.; Zhang, W.; Jiang, W.; Xiao, X.; Chen, S.; Dai, C.; Cong, Z.; Ji, Z.; et al. Poly(2-Oxazoline)-Based Functional Peptide Mimics: Eradicating MRSA Infections and Persisters while Alleviating Antimicrobial Resistance. Angew. Chem. Int. Ed. 2020, 59, 6412–6419. [Google Scholar] [CrossRef]
- Hoogenboom, R. Poly(2-oxazoline)s: A Polymer Class with Numerous Potential Applications. Angew. Chem. Int. Ed. 2009, 48, 7978–7994. [Google Scholar] [CrossRef] [PubMed]
- Fik, C.P.; Krumm, C.; Muennig, C.; Baur, T.I.; Salz, U.; Bock, T.; Tiller, J.C. Impact of Functional Satellite Groups on the Antimicrobial Activity and Hemocompatibility of Telechelic Poly(2-methyloxazoline)s. Biomacromolecules 2012, 13, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Krumm, C.; Harmuth, S.; Hijazi, M.; Neugebauer, B.; Kampmann, A.L.; Geltenpoth, H.; Sickmann, A.; Tiller, J.C. Antimicrobial Poly(2-methyloxazoline)s with Bioswitchable Activity through Satellite Group Modification. Angew. Chem. Int. Ed. 2014, 53, 3830–3834. [Google Scholar] [CrossRef]
- Benski, L.; Tiller, J.C. Telechelic Biocidal Poly(2-oxazoline)s and Polycations. Eur. Polym. J. 2019, 120, 109233. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, M.; Cong, Z.; Xie, J.; Zhang, W.; Chen, S.; Zou, J.; Ji, Z.; Shao, N.; Chen, X.; et al. Short Guanidinium-Functionalized Poly(2-oxazoline)s Displaying Potent Therapeutic Efficacy on Drug-Resistant Fungal Infections. Angew. Chem. Int. Ed. 2022, 61, e202200778. [Google Scholar]
- Kelly, A.M.; Kaltenhauser, V.; Muhlbacher, I.; Rametsteiner, K.; Kren, H.; Slugovc, C.; Stelzer, F.; Wiesbrock, F. Poly(2-oxazoline)-derived contact biocides: Contributions to the understanding of antimicrobial activity. Macromol. Biosci. 2013, 13, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qian, X.; Zhang, Z.; Li, C.; Xie, C.; Wu, W.; Jiang, X. Supramolecular Amphiphilic Polymer-Based Micelles with Seven-Armed Polyoxazoline Coating for Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 5768–5777. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, C.; Zhou, S.; Wu, W.; Jiang, X. Target-Amplified Drug Delivery of Polymer Micelles Bearing Staudinger Ligation. ACS Appl. Mater. Interfaces 2019, 11, 32697–32705. [Google Scholar] [CrossRef]
- Schmidt, M.; Harmuth, S.; Barth, E.R.; Wurm, E.; Fobbe, R.; Sickmann, A.; Krumm, C.; Tiller, J.C. Conjugation of Ciprofloxacin with Poly(2-oxazoline)s and Polyethylene Glycol via End Groups. Bioconjug. Chem. 2015, 26, 1950–1962. [Google Scholar] [CrossRef]
- Chen, W.; Su, L.; Zhang, P.; Li, C.; Zhang, D.; Wu, W.; Jiang, X. Thermo and pH dual-responsive drug-linked pseudo-polypeptide micelles with a comb-shaped polymer as a micellar exterior. Polym. Chem. 2017, 8, 6886–6894. [Google Scholar] [CrossRef]
- Schmidt, M.; Bast, L.K.; Lanfer, F.; Richter, L.; Hennes, E.; Seymen, R.; Krumm, C.; Tiller, J.C. Poly(2-oxazoline)-Antibiotic Conjugates with Penicillins. Bioconjug. Chem. 2017, 28, 2440–2451. [Google Scholar] [CrossRef]
- Sedlacek, O.; Monnery, B.D.; Mattova, J.; Kucka, J.; Panek, J.; Janouskova, O.; Hocherl, A.; Verbraeken, B.; Vergaelen, M.; Zadinova, M.; et al. Poly(2-ethyl-2-oxazoline) Conjugates with Doxorubicin for Cancer Therapy: In vitro and in vivo Evaluation and Direct Comparison to Poly[N-(2-hydroxypropyl)methacrylamide] Analogues. Biomaterials 2017, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Romanovska, A.; Wolf, Y.; Nguyen, T.-D.; Krupp, A.; Tumbrink, H.L.; Lategahn, J.; Volmer, J.; Rauh, D.; Luetz, S.; et al. Insights into the Kinetics of the Resistance Formation of Bacteria against Ciprofloxacin Poly(2-methyl-2-oxazoline) Conjugates. Bioconjug. Chem. 2018, 29, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, Y.; Sedlacek, O.; Van Guyse, J.F.R.; Bender, J.; Zhong, Z.; De Geest, B.G.; Hoogenboom, R. Poly(2-ethyl-2-oxazoline) Conjugates with Salicylic Acid via Degradable Modular Ester Linkages. Biomacromolecules 2020, 21, 3207–3215. [Google Scholar] [CrossRef]
- Park, J.R.; Verderosa, A.D.; Totsika, M.; Hoogenboom, R.; Dargaville, T.R. Thermoresponsive Polymer-Antibiotic Conjugates Based on Gradient Copolymers of 2-Oxazoline and 2-Oxazine. Biomacromolecules 2021, 22, 5185–5194. [Google Scholar] [CrossRef]
- Christova, D.; Velichkova, R.; Loos, W.; Goethals, E.J.; Prez, F.D. New thermo-responsive polymer materials based on poly(2-ethyl-2-oxazoline) segments. Polymer 2003, 44, 2255–2261. [Google Scholar] [CrossRef]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological Overviews from Bench to Bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Pasut, G.; Via, L.D.; Fijten, M.W.M.; Schubert, U.S.; Hoogenboom, R.; Veronese, F.M. Synthesis and characterization of poly(2-ethyl 2-oxazoline)-conjugates with proteins and drugs: Suitable alternatives to PEG-conjugates? J. Control. Release 2008, 125, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Li, C.; Wang, D.; Gao, Y.; Zhang, C.; Zhao, L.; Li, Y.; Liu, Y.; Li, X. Poly(2-ethyl-2-oxazoline)-doxorubicin Conjugate-based Dual Endosomal pH-sensitive Micelles with Enhanced Antitumor Efficacy. Bioconjug. Chem. 2015, 26, 110–119. [Google Scholar] [CrossRef]
- Rajendran, D.; Elizabeth, J.; Manoharan, S.; Vellala, N.; Kootallur, B.; Paul, P.M.; Angamuthu, A.; Bhagavathsingh, J. Synthesis, Characterization and Relaxivity Validations of Gd(III) Complex of DOTA Tetrahydrazide as MRI Contrast Agent. J. Mol. Struct. 2022, 1255, 132474. [Google Scholar] [CrossRef]
- Shao, N.; Yuan, L.; Ma, P.; Zhou, M.; Xiao, X.; Cong, Z.; Wu, Y.; Xiao, G.; Fei, J.; Liu, R. Heterochiral β-Peptide Polymers Combating Multidrug-Resistant Cancers Effectively without Inducing Drug Resistance. J. Am. Chem. Soc. 2022, 144, 7283–7294. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Yan, Y.; Lv, W.; Li, Z.; Wang, X.; Tao, Y. Anion-binding catalysis enables living cationic polymerization. Nat. Synth. 2022, 1, 815–823. [Google Scholar] [CrossRef]
- Hu, F.; Xie, S.; Jiang, L.; Shen, Z. Living cationic ring-opening polymerization of 2-oxazolines initiated by rare-earth metal triflates. RSC Adv. 2014, 4, 59917–59926. [Google Scholar] [CrossRef]
- Zhou, M.; Xiao, X.; Cong, Z.; Wu, Y.; Zhang, W.; Ma, P.; Chen, S.; Zhang, H.; Zhang, D.; Zhang, D.; et al. Water-Insensitive Synthesis of Poly-beta-Peptides with Defined Architecture. Angew. Chem. Int. Ed. 2020, 59, 7240–7244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Wang, S.; Tao, Y.; Wang, X. S-Carboxyanhydrides: Ultrafast and Selective Ring-Opening Polymerizations Towards Well-defined Functionalized Polythioesters. Angew. Chem. Int. Ed. 2021, 60, 10798–10805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zou, J.; Liu, L.; Xiao, X.; Deng, S.; Wu, Y.; Xie, J.; Cong, Z.; Ji, Z.; Liu, R. Synthesis of poly-alpha/beta-peptides with tunable sequence via the copolymerization on N-carboxyanhydride and N-thiocarboxyanhydride. iScience 2021, 24, 103124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hu, X.; Song, F.; Liu, Z.; Xie, Z.; Jing, X. Studies on the Biological Character of a New pH-sensitive Doxorubicin Prodrug with Tumor Targeting Using a LC-MS/MS Method. Anal. Chem. 2014, 6, 3159. [Google Scholar] [CrossRef]
- Xie, Y.; Shao, N.; Jin, Y.; Zhang, L.; Jiang, H.; Xiong, N.; Su, F.; Xu, H. Determination of Non-liposomal and Liposomal Doxorubicin in Plasma by LC-MS/MS Coupled with an Effective Solid Phase Extraction: In Comparison with Ultrafiltration Technique and Application to a Pharmacokinetic Study. J. Chromatogr. B 2018, 1072, 149–160. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Cui, R.; Luo, Z.; Cong, Z.; Shao, N.; Yuan, L.; Gu, J.; He, H.; Liu, R. Convenient and Controllable Synthesis of Poly(2-oxazoline)-Conjugated Doxorubicin for Regulating Anti-Tumor Selectivity. J. Funct. Biomater. 2023, 14, 382. https://doi.org/10.3390/jfb14070382
Zhou M, Cui R, Luo Z, Cong Z, Shao N, Yuan L, Gu J, He H, Liu R. Convenient and Controllable Synthesis of Poly(2-oxazoline)-Conjugated Doxorubicin for Regulating Anti-Tumor Selectivity. Journal of Functional Biomaterials. 2023; 14(7):382. https://doi.org/10.3390/jfb14070382
Chicago/Turabian StyleZhou, Min, Ruxin Cui, Zhengjie Luo, Zihao Cong, Ning Shao, Ling Yuan, Jiawei Gu, Hongyan He, and Runhui Liu. 2023. "Convenient and Controllable Synthesis of Poly(2-oxazoline)-Conjugated Doxorubicin for Regulating Anti-Tumor Selectivity" Journal of Functional Biomaterials 14, no. 7: 382. https://doi.org/10.3390/jfb14070382