Bioactive Glass-Ceramic Scaffolds Coated with Hyaluronic Acid–Fatty Acid Conjugates: A Feasibility Study †
Abstract
:1. Introduction
2. Results and Discussion
2.1. HA–Fatty-Acid Conjugates
2.2. Coating Procedure of the HA-Based Conjugates on Bioactive Glass Scaffolds
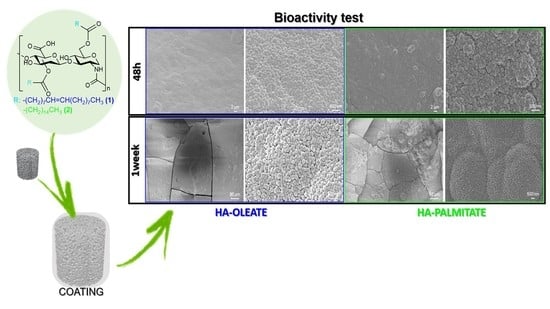
2.3. Conjugate-Coated Bioactive Glass Scaffolds
3. Materials and Methods
3.1. Starting Reagents
3.2. Synthesis and Characterization of HA-Fatty Acids Conjugates
3.2.1. Synthesis of HA–Fatty-Acid Conjugates
3.2.2. FT-IR Characterization
3.3. Fabrication and Characterization of Glass-Derived Scaffolds
3.4. Coating of HA–Fatty-Acid Conjugates on Bioactive Glass
3.5. Decoating of HA–Fatty-Acid Conjugates with Bioactive Glass
3.6. In Vitro Bioactivity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, P.C.; Mikos, A.G.; Fisher, J.P.; Jansen, J.A. Strategic directions in tissue engineering. Tissue Eng. 2007, 13, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Hoppe, A.; Guldal, N.; Boccaccini, A.R. Biological response to ionic dissolution products from bioactive glass and glass-ceramics in the context of bone tissue engineering. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.; Mozafari, M. Bioactive glasses: Sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive glasses: From parent 45S5 composition to scaffold-assisted tissue-healing therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- El-Rashidy, A.; Roether, J.; Harhaus, L.; Kneser, U.; Boccaccini, A. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hill, R.; Milan, P.B.; Joghataei, M.T.; Hamzehlou, S.; Baino, F. Synergistic combination of bioactive glasses and polymers for enhanced bone tissue regeneration. Mater. Today Proc. 2018, 5, 15532–15539. [Google Scholar] [CrossRef]
- Bedi, A.; Manjoo, A.; Niazi, F.; Shaw, P.; Mease, P. Anti-Inflammatory Effects of Intra-Articular Hyaluronic Acid: A Systematic Review. Clin. Pap. 2019, 10, 43–52. [Google Scholar]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Franco Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.; Wu, C.; Dubey, N.K.; Lo, W.; Tsai, F.; Tung, T.D.X.; Hung, W.; Hsu, W.; Chen, W.; Deng, W. Mechanistic insight into hyaluronic acid and platelet-rich plasma-mediated anti-inflammatory and anti-apoptotic activities in osteoarthritic mice. Aging 2018, 10, 4152–4165. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Calce, E.; Ringhieri, P.; Mercurio, F.A.; Leone, M.; Bugatti, V.; Saviano, M.; Vittoria, V.; De Luca, S. A biocompatible process to prepare hyaluronan- based material able to self-assemble into stable nano-particles. RSC Adv. 2015, 5, 29573. [Google Scholar] [CrossRef]
- Pepe, G.; Calce, E.; Verdoliva, V.; Saviano, M.; Maglione, V.; Di Pardo, A.; De Luca, S. Curcumin-Loaded Nanoparticles Based on Amphiphilic Hyaluronan-Conjugate Explored as Targeting Delivery System for Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 8846. [Google Scholar] [CrossRef]
- Calce, E.; Mercurio, F.A.; Leone, M.; Saviano, M.; De Luca, S. Eco-friendly microwave-assisted protocol to prepare hyaluronan-fattyacid conjugates and to induce their self-assembly process. Carbohydr. Polym. 2016, 143, 84–89. [Google Scholar] [CrossRef]
- Calce, E.; Petricci, E.; Saviano, M.; De Luca, S. Green microwave-assisted procedure to generate bio-based pectin materials. Sustain. Chem. Pharm. 2017, 5, 127–130. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Tugarova, A.V.; Dyatlova, Y.A.; Tarantilis, P.A.; Grigoryeva, O.P.; Fainleib, A.M.; De Luca, S. Methodological effects in Fourier transform infrared (FTIR) spectroscopy: Implications for structural analyses of biomacromolecular samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 19, 3558–3564. [Google Scholar] [CrossRef]
- Fiume, E.; Ciavattini, S.; Verné, E.; Baino, F. Foam replica method in the manufacturing of bioactive glass scaffolds: Out-of-date technology or still underexploited potential? Materials 2021, 14, 2795. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Fiume, E.; Schiavi, A.; Orlygsson, G.; Bignardi, C.; Verné, E.; Baino, F. Comprehensive assessment of bioactive glass and glass-ceramic scaffold permeability: Experimental measurements by pressure wave drop, modelling and computed tomography-based analysis. Acta Biomater. 2021, 119, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Serino, G.; Bignardi, C.; Verné, E.; Baino, F. Bread-derived bioactive porous scaffolds: An innovative and sustainable approach to bone tissue engineering. Molecules 2019, 24, 2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiume, E.; Tulyaganov, D.; Ubertalli, G.; Verné, E.; Baino, F. Dolomite-foamed bioactive silicate scaffolds for bone tissue repair. Materials 2020, 13, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Bretcanu, O.; Chatzistavrou, X.; Paraskevopolous, K.; Conradt, R.; Thompson, I.; Boccaccini, A.R. Sintering and crystallisation of 45S5 Bioglass® powder. J. Eur. Ceram. Soc. 2009, 29, 3299–3306. [Google Scholar] [CrossRef]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and bioactive glasses and their impact on healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Fiume, E.; Tulyaganov, D.U.; Akbarov, A.; Ziyadullaeva, N.; Cochis, A.; Scalia, A.C.; Rimondini, L.; Verné, E.; Baino, F. Biological evaluation of a new sodium-potassium silico-phosphate glass for bone regeneration: In vitro and in vivo studies. Materials 2021, 14, 4546. [Google Scholar] [CrossRef]
- Greenspan, D.C. Bioactive glass: Mechanisms of bone bonding. Tandläkartidningen Ǻrk 1999, 91, 1–32. [Google Scholar]
- Kohn, D.H.; Sarmadi, M.; Helman, J.I.; Krebsbach, P.H. Effects of pH on human bone marrow stromal cells in vitro: Implications for tissue engineering of bone. J. Biomed. Mater. Res. 2002, 60, 2–9. [Google Scholar] [CrossRef] [Green Version]
- López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regi, M. Ordered mesoporous bioactive glasses for bone tissue regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Baino, F.; Marshall, M.; Kirk, N.; Vitale-Brovarone, C. Design, selection and characterization of novel glasses and glass-ceramics for use in prosthetic applications. Ceram. Int. 2016, 42, 1482–1491. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Miola, M.; Leone, F.; Onida, B.; Verné, E. Fe-doped bioactive glass-derived scaffolds produced by sol-gel foaming. Mater. Lett. 2019, 235, 207–211. [Google Scholar] [CrossRef]
- Tulyaganov, D.; Fiume, E.; Akbarov, A.; Ziyadullaeva, N.; Murtazaev, S.; Rahdar, A.; Massera, J.; Verné, E.; Baino, F. In vivo evaluation of 3D-printed silica-based bioactive glass scaffolds for bone regeneration. J. Funct. Biomater. 2022, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Banijamali, S.; Baino, F.; Kargozar, S.; Hill, R.G. Calcium carbonate: Adored and ignored in bioactivity assessment. Acta Biomater. 2019, 91, 35–47. [Google Scholar] [CrossRef]
- Fiume, E.; Serino, G.; Bignardi, C.; Verné, E.; Baino, F. Sintering behavior of a six-oxide silicate bioactive glass for scaffold manufacturing. Appl. Sci. 2020, 10, 8279. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, S.; Verdoliva, V.; Kargozar, S.; Baino, F. Bioactive Glass-Ceramic Scaffolds Coated with Hyaluronic Acid–Fatty Acid Conjugates: A Feasibility Study. J. Funct. Biomater. 2023, 14, 26. https://doi.org/10.3390/jfb14010026
De Luca S, Verdoliva V, Kargozar S, Baino F. Bioactive Glass-Ceramic Scaffolds Coated with Hyaluronic Acid–Fatty Acid Conjugates: A Feasibility Study. Journal of Functional Biomaterials. 2023; 14(1):26. https://doi.org/10.3390/jfb14010026
Chicago/Turabian StyleDe Luca, Stefania, Valentina Verdoliva, Saeid Kargozar, and Francesco Baino. 2023. "Bioactive Glass-Ceramic Scaffolds Coated with Hyaluronic Acid–Fatty Acid Conjugates: A Feasibility Study" Journal of Functional Biomaterials 14, no. 1: 26. https://doi.org/10.3390/jfb14010026