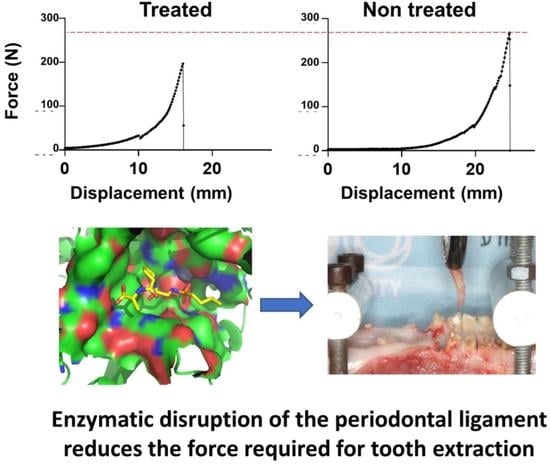
Collagenase Administration into Periodontal Ligament Reduces the Forces Required for Tooth Extraction in an Ex situ Porcine Jaw Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression and Purification of Collagenase G
2.2. Experimental Set-Up and Jaw Preparation
2.3. Injection of Collagenase into the PDL
2.4. Mounting of Porcine Mandibles to the Loading Machine
2.5. Measurement of Force Required for Root Extraction
2.6. Cellular Viability Assay
2.7. Cellular Toxicity
2.8. Data Analysis
3. Results
3.1. Experimental Setup
3.2. Qualitative and Quantitative Analysis of Extraction Forces
3.3. Evaluation of Cellular Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, A. Principles and Techniques of Exodontia; Springer: Singapore, 2021; pp. 259–297. [Google Scholar]
- Badwal, R.S.; Emery, A. Uncomplicated Exodontia. In Evidence-Based Oral Surgery: A Clinical Guide for the General Dental Practitioner; Ferneini, E.M., Goupil, M.T., Eds.; Springer International Publishing: Cham, Swizterland, 2019; pp. 151–171. [Google Scholar]
- McKenzie, W.S. Principles of Exodontia. Oral Maxillofac. Surg. Clin. N. Am. 2020, 32, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.L. Some complications of tooth extraction: Lectures delivered at the Royal College of Surgeons of England on 27 April 1961. Ann. R. Coll. Surg. Engl. 1962, 30, 309–323. [Google Scholar] [PubMed]
- Anzai, H.; Mitate, E.; Tanaka, F.; Oobayashi, Y.; Yoshizumi, J.; Hiraki, A. Unexpected hemorrhage during wisdom tooth extraction: Report of two cases and review of management techniques. Oral Sci. Int. 2020, 17, 190–195. [Google Scholar] [CrossRef]
- Jambhekar, S.; Kernen, F.; Bidra, A.S. Clinical and histologic outcomes of socket grafting after flapless tooth extraction: A systematic review of randomized controlled clinical trials. J. Prosthet. Dent. 2015, 113, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.O.; Blanchette, D.; Dawson, D.V. Effect of Alveolar Ridge Preservation after Tooth Extraction: A Systematic Review and Meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef]
- Bouloux, G.F.; Steed, M.B.; Perciaccante, V.J. Complications of third molar surgery. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 117–128. [Google Scholar] [CrossRef]
- Robinson, P.P.; Loescher, A.R.; Yates, J.M.; Smith, K.G. Current management of damage to the inferior alveolar and lingual nerves as a result of removal of third molars. Br. J. Oral Maxillofac. Surg. 2004, 42, 285–292. [Google Scholar] [CrossRef]
- Papi, P.; Rosella, D.; Giardino, R.; Cicalini, E.; Piccoli, L.; Pompa, G. Medication-related osteonecrosis of the jaw: Clinical and practical guidelines. J. Int. Soc. Prev. Commun. Dent. 2016, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Shibahara, T. Antiresorptive Agent-Related Osteonecrosis of the Jaw (ARONJ): A Twist of Fate in the Bone. Tohoku J. Exp. Med. 2019, 247, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Blus, C.; Szmukler-Moncler, S. Atraumatic tooth extraction and immediate implant placement with Piezosurgery: Evaluation of 40 sites after at least 1 year of loading. Int. J. Periodontics Restor. Dent. 2010, 30, 355–363. [Google Scholar]
- Tsirlis, A.T.; Eliades, A.N.; Georgopoulou-Karanikola, T.G.; Vasiloudi, M.I. A technique for atraumatic root extraction, immediate implant placement and loading in maxillary aesthetic zone. Oral Surg. 2015, 8, 102–110. [Google Scholar] [CrossRef]
- El-Kenawy, M.H.; Ahmed, W.M.S. Comparison Between Physics and Conventional Forceps in Simple Dental Extraction. J. Maxillofac. Oral Surg. 2015, 14, 949–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muska, E.; Walter, C.; Knight, A.; Taneja, P.; Bulsara, Y.; Hahn, M.; Desai, M.; Dietrich, T. Atraumatic vertical tooth extraction: A proof of principle clinical study of a novel system. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e303–e310. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Stern, A.; Dym, H. Technological advances in extraction techniques and outpatient oral surgery. Dent. Clin. N. Am. 2011, 55, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Told, R.; Marada, G.; Rendeki, S.; Pentek, A.; Nagy, B.; Molnar, F.J.; Maroti, P. Manufacturing a First Upper Molar Dental Forceps Using Continuous Fiber Reinforcement (CFR) Additive Manufacturing Technology with Carbon-Reinforced Polyamide. Polymers 2021, 13, 2647. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Vidya, B.; Alexander, M.; Deshmukh, S. Periotome as an Aid to Atraumatic Extraction: A Comparative Double Blind Randomized Controlled Trial. J. Maxillofac. Oral Surg. 2015, 14, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.K. Using the Piezosurgery device: Basics and possibilities. Dent Implant. Update 2007, 18, 1–4. [Google Scholar]
- Schlee, M.; Steigmann, M.; Bratu, E.; Garg, A.K. Piezosurgery: Basics and possibilities. Implant Dent. 2006, 15, 334–340. [Google Scholar] [CrossRef]
- Hong, B.S.; Bulsara, Y.; Gorecki, P.; Dietrich, T. Minimally invasive vertical versus conventional tooth extraction An interrupted time series study. J. Am. Dent. Assoc. 2018, 149, 688–695. [Google Scholar] [CrossRef] [Green Version]
- Kawada, J.; Komatsu, K. In Vitro Effects of Collagenase on Biomechanical Properties and Morphological Features of the Rat Molar Periodontal Ligament. Jpn. J. Oral Biol. 2000, 42, 193–205. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Balakireva, A.V.; Kuznetsova, N.V.; Vukolova, M.N.; Litvitsky, P.F.; Zamyatnin, A.A., Jr. Collagenolytic Enzymes and their Applications in Biomedicine. Curr. Med. Chem 2019, 26, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.S.; Correia, A.; Esteves, A.C. Bacterial collagenases—A review. Crit. Rev. Microbiol. 2016, 42, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Smeraglia, F.; Del Buono, A.; Maffulli, N. Collagenase clostridium histolyticum in Dupuytren’s contracture: A systematic review. Br. Med. Bull. 2016, 118, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrielson, A.T.; Spitz, J.T.; Hellstrom, W.J.G. Collagenase Clostridium Histolyticum in the Treatment of Urologic Disease: Current and Future Impact. Sex Med. Rev. 2018, 6, 143–156. [Google Scholar] [CrossRef]
- Warwick, D.; Arandes-Renú, J.M.; Pajardi, G.; Witthaut, J.; Hurst, L.C. Collagenase Clostridium histolyticum:emerging practice patterns and treatment advances. J. Plast. Surg. Hand Surg. 2016, 50, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, K.; Shibata, T.; Shimada, A. Analysis of contribution of collagen fibre component in viscoelastic behaviour of periodontal ligament using enzyme probe. J. Biomech. 2007, 40, 2700–2706. [Google Scholar] [CrossRef]
- Tohar, R.; Ansbacher, T.; Sher, I.; Afriat-Jurnou, L.; Weinberg, E.; Gal, M. Screening Collagenase Activity in Bacterial Lysate for Directed Enzyme Applications. Int. J. Mol. Sci. 2021, 22, 8552. [Google Scholar] [CrossRef]
- Hay-Koren, A.; Caspi, M.; Zilberberg, A.; Rosin-Arbesfeld, R. The EDD E3 ubiquitin ligase ubiquitinates and up-regulates beta-catenin. Mol. Biol. Cell 2011, 22, 399–411. [Google Scholar] [CrossRef]
- Štembírek, J.; Kyllar, M.; Putnová, I.; Stehlík, L.; Buchtová, M. The pig as an experimental model for clinical craniofacial research. Lab. Anim. 2012, 46, 269–279. [Google Scholar] [CrossRef]
- Dietrich, T.; Schmid, I.; Locher, M.; Addison, O. Extraction force and its determinants for minimally invasive vertical tooth extraction. J. Mech. Behav. Biomed. Mater. 2020, 105, 103711. [Google Scholar] [CrossRef]
- Syed, F.; Thomas, A.N.; Singh, S.; Kolluru, V.; Emeigh Hart, S.G.; Bayat, A. In vitro study of novel collagenase (XIAFLEX®) on Dupuytren’s disease fibroblasts displays unique drug related properties. PLoS ONE 2012, 7, e31430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renton, T.; Woolcombe, S.; Taylor, T.; Hill, C.M. Oral surgery: Part 1. Introduction and the management of the medically compromised patient. Br. Dent. J. 2013, 215, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kün-Darbois, J.D.; Fauvel, F. Medication-related osteonecrosis and osteoradionecrosis of the jaws: Update and current management. Morphologie 2021, 105, 170–187. [Google Scholar] [CrossRef] [PubMed]
- Touyz, L.Z.; Afrashtehfar, K.I. Cryogenically salvaged teeth as a potential source for grafting dentoalveolar, periodontal or maxillofacial defects. Med. Hypotheses 2016, 92, 28–30. [Google Scholar] [CrossRef]
- Atkinson, H.F.; Ralph, W.J. In Vitro Strength of the Human Periodontal Ligament. J. Dent. Res. 1977, 56, 48–52. [Google Scholar] [CrossRef]
- Chiba, M.; Ohkawa, S. Measurement of the tensile strength of the periodontium in the rat mandibular first molar. Arch. Oral Biol. 1980, 25, 569–572. [Google Scholar] [CrossRef]
- Chiba, M.; Ohshima, S.; Takizawa, K. Measurement of the force required to extract the mandibular incisor of rats of various ages. Arch. Oral Biol. 1980, 25, 683–687. [Google Scholar] [CrossRef]
- Komatsu, K. Mechanical Strength and Viscoelastic Response of the Periodontal Ligament in Relation to Structure. J. Dent. Biomech. 2010, 1, 502318. [Google Scholar] [CrossRef]
- Komatsu, K.; Ohshima, S.; Chiba, M. Measurement of the force required to extract the mandibular first molar from its socket in the dissected jaw of growing young rats. Gerodontology 1990, 9, 3–7. [Google Scholar] [CrossRef]
- Ohshima, S.; Nakamura, G.; Chiba, M. Effects of lathyrogens on the mechanical strength of the periodontal ligament in the rat mandibular first molar. J. Periodontal Res. 1989, 24, 343–350. [Google Scholar] [CrossRef]
- Xia, Z.; Jiang, F.; Chen, J. Estimation of periodontal ligament’s equivalent mechanical parameters for finite element modeling. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 486–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picton, D.C.; Wills, D.J. Viscoelastic properties of the periodontal ligament and mucous membrane. J. Prosthet. Dent. 1978, 40, 263–272. [Google Scholar] [CrossRef]
- Datta, S.; Rajnish, K.N.; George Priya Doss, C.; Melvin Samuel, S.; Selvarajan, E.; Zayed, H. Enzyme therapy: A forerunner in catalyzing a healthy society? Expert Opin. Biol. Ther. 2020, 20, 1151–1174. [Google Scholar] [CrossRef] [PubMed]
- Anbu, P.; Gopinath, S.C.B.; Cihan, A.C.; Chaulagain, B.P. Microbial Enzymes and Their Applications in Industries and Medicine. BioMed Res. Int. 2013, 2013, 1–2. [Google Scholar] [CrossRef]
- Zinger, A.; Adir, O.; Alper, M.; Simon, A.; Poley, M.; Tzror, C.; Yaari, Z.; Krayem, M.; Kasten, S.; Nawy, G.; et al. Proteolytic Nanoparticles Replace a Surgical Blade by Controllably Remodeling the Oral Connective Tissue. ACS Nano 2018, 12, 1482–1490. [Google Scholar] [CrossRef]
- Ujiie, Y.; Shimada, A.; Komatsu, K.; Gomi, K.; Oida, S.; Arai, T.; Fukae, M. Degradation of noncollagenous components by neutrophil elastase reduces the mechanical strength of rat periodontal ligament. J. Periodontal Res. 2008, 43, 22–31. [Google Scholar] [CrossRef]
- Hoppe, I.J.; Brandstetter, H.; Schonauer, E. Biochemical characterisation of a collagenase from Bacillus cereus strain Q1. Sci. Rep. 2021, 11, 4187. [Google Scholar] [CrossRef]
- Eckhard, U.; Schonauer, E.; Ducka, P.; Briza, P.; Nuss, D.; Brandstetter, H. Biochemical characterization of the catalytic domains of three different Clostridial collagenases. Biol. Chem. 2009, 390, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Abfalter, C.M.; Schönauer, E.; Ponnuraj, K.; Huemer, M.; Gadermaier, G.; Regl, C.; Briza, P.; Ferreira, F.; Huber, C.G.; Brandstetter, H.; et al. Cloning, Purification and Characterization of the Collagenase ColA Expressed by Bacillus cereus ATCC 14579. PLoS ONE 2016, 11, e0162433. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohar, R.; Alali, H.; Ansbacher, T.; Brosh, T.; Sher, I.; Gafni, Y.; Weinberg, E.; Gal, M. Collagenase Administration into Periodontal Ligament Reduces the Forces Required for Tooth Extraction in an Ex situ Porcine Jaw Model. J. Funct. Biomater. 2022, 13, 76. https://doi.org/10.3390/jfb13020076
Tohar R, Alali H, Ansbacher T, Brosh T, Sher I, Gafni Y, Weinberg E, Gal M. Collagenase Administration into Periodontal Ligament Reduces the Forces Required for Tooth Extraction in an Ex situ Porcine Jaw Model. Journal of Functional Biomaterials. 2022; 13(2):76. https://doi.org/10.3390/jfb13020076
Chicago/Turabian StyleTohar, Ran, Hen Alali, Tamar Ansbacher, Tamar Brosh, Inbal Sher, Yossi Gafni, Evgeny Weinberg, and Maayan Gal. 2022. "Collagenase Administration into Periodontal Ligament Reduces the Forces Required for Tooth Extraction in an Ex situ Porcine Jaw Model" Journal of Functional Biomaterials 13, no. 2: 76. https://doi.org/10.3390/jfb13020076