Bioaccumulation of Metals/Metalloids and Histological and Immunohistochemical Changes in the Tissue of the European Hake, Merluccius merluccius (Linnaeus, 1758) (Pisces: Gadiformes: Merlucciidae), for Environmental Pollution Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metals and Metalloids Analysis
2.2. Histological Analysis
2.3. Immunohistochemical Analysis
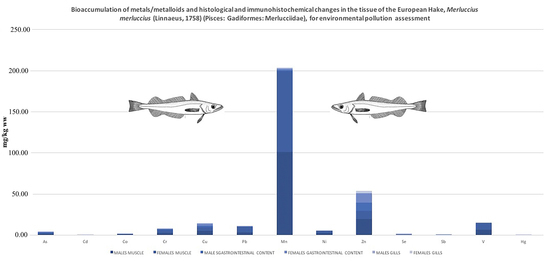
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Focardi, S.; Gavilán, J.F.; Barra, R.; Fossi, M.C.; Casini, S.; Salinas, G.; Parra, O. Biochemical biomarkers in fish from different river systems reflect exposure to a variety of anthropogenic stressors. Bull. Environ. Contam. Toxicol. 2001, 66, 476–483. [Google Scholar]
- Di Domenico, A.; Miniero, R. Persistent organic micropollutants in Mediterranean organisms and risk associated. Ann. Dell’istituto Super. Di Sanita 2003, 39, 1–5. [Google Scholar]
- Storelli, M.M.; Marcotrigiano, G.O. Bioindicator organisms: Heavy metal pollution evaluation in the Ionian Sea (Mediterranea Sea–Italy). Environ. Monit. Assess. 2005, 102, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Wayland, M. Metals as threats to wildlife. In Short Course on Wildlife Toxicology; Canadian Cooperative Wildlife Centre, Western College of Veterinary Medicine, University of Saskatchewan: Saskatoon, SK, Canada, 2000. [Google Scholar]
- Riggio, M.; Trinchella, F.; Filosa, S.; Parisi, E.; Scudiero, R. Accumulation of zinc, copper, and metallothionein mRNA in lizard ovary proceeds without a concomitant increase in metallothionein content. Mol. Reprod. Dev. 2003, 66, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Hanas, J.S.; Hazuda, D.J.; Bogenhagen, D.F.; Wu, F.Y.; Wu, C.W. Xenopus transcription factor A requires zinc for binding to the 5S RNA gene. J. Biol. Chem. 1983, 258, 14120–14125. [Google Scholar]
- Grummt, I.; Kuhn, A.; Bartsch, I.; Rosenbauer, H. A transcription terminator located upstream of the mouse rDNA initiation site affects rRNA synthesis. Cell 1983, 47, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Dixon, M.; Webb, E.C. Enzyme inhibition and activation. In Enzymes; Dixon, M., Webb, E.C., Eds.; Academic Press: New York, NY, USA, 1979; p. 332. [Google Scholar]
- Bryce-Smith, D. Zinc deficiency-the neglected factor. Chem. Br. 1989, 25, 783–786. [Google Scholar]
- Cousins, R.J. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ruloplasmin. Physiol. Rev. 1985, 2, 238–309. [Google Scholar] [CrossRef]
- Guillou, M.; Ouiniou, F.; Huart, B.; Pagano, G. Comparision of embryonic development and metal contamination in several populations of the sea urchin Sphaerechinus granularis (Lamark) exposed to anthropogenic pollution. Arch. Environ. Contam. Toxicol. 2000, 39, 337–344. [Google Scholar] [CrossRef]
- Au, D.W.; Reunov, A.A.; Wu, R.S. Reproductive impairment of sea urchin upon chronic exposure to cadmium. Part II: Effects on sperm development. Environ. Pollut. 2001, 111, 11–20. [Google Scholar] [CrossRef]
- Sunderman, F.W., Jr.; Plowman, M.C.; Kroftova, O.S.; Grbac-Ivankoyic, S.; Foglia, L.; Crivello, J.F. Effects of teratogenic exposures to Zn2+, Cd2+, Ni2+, Co2+, and Cu2+ on metallothionein mRNA contents of Xenopus embryos. Pharmcol. Toxicol. 1995, 76, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Hanna, L.A.; Peters, J.M.; Wiley, L.M.; Clegg, M.S.; Kenn, C.L. Comparative effects of essential and nonessential metals on preimplantation mouse embryo development in vitro. Toxicology 1997, 116, 123–131. [Google Scholar] [CrossRef]
- Calevro, F.; Beyrsmann, D.; Hartwig, A. Effect of cadmium (II) on the extent of oxidative DNA damage in primary brain cell cultures from Pleurodeles larvae. Toxicol. Lett. 1998, 94, 217–225. [Google Scholar] [CrossRef]
- Oskarsson, A.; Palmiger Hallén, I.; Sundberg, J.; Petersson Gravé, K. Risk assessment in relation to neonatal metal exposure. Analyst 1998, 123, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, K.; Karkoszka, J.; Cohen, B.; Baranski, B.; Jakubowski, M.; Cosma, G.; Taioli, E.; Toniolo, P. Occupational exposures to Cd, Ni, and Cr modulate titers of antioxidized DNA base autoantibodies. Environ. Health Perspect. 1994, 102, 221–225. [Google Scholar]
- Hartwing, A. Role of DNA repair inhibition in lead and cadmium induced genotoxicity a: Review. Environ. Health Perspect. 1994, 102, 45–50. [Google Scholar]
- Ferrara, F.; Funari, E. Chemical Risk Associated with the Water Quality of the Adriatic Sea; ISTISAN 04/4 Reports; Final Report of the Activities Financed by the MURST/CNR “PRISMA2” Project; Istituto Superiore di Sanità: Rome, Italy, 2004. [Google Scholar]
- Carrozzi, V.; Di Lorenzo, M.; Massi, D.; Titone, A.; Ardizzone, G.; Colloca, F. Prey preferences and ontogenetic diet shift of European hake Merluccius merluccius (Linnaeus, 1758) in the central Mediterranean Sea. Reg. Stud. Mar. Sci. 2019, 25, 100440. [Google Scholar] [CrossRef]
- Martínez-Morcillo, S.; Pérez-López, M.; Míguez, M.P.; Valcárcel, Y.; Soler, F. Comparative study of esterase activities in different tissues of marine fish species Trachurus trachurus, Merluccius merluccius and Trisopterus luscus. Sci. Total Environ. 2019, 679, 12–22. [Google Scholar] [CrossRef]
- Copat, C.; Rizzo, M.; Zuccaro, A.; Grasso, A.; Zuccarello, P.; Fiore, M.; Mancini, G.; Ferrante, M. Metals/Metalloids and Oxidative Status Markers in Saltwater Fish from the Ionic Coast of Sicily, Mediterranean Sea. Int. J. Environ. Res. 2020, 14, 15–27. [Google Scholar] [CrossRef]
- Copat, C.; Grasso, A.; Fiore, M.; Cristaldi, A.; Zuccarello, P.; Signorelli, SS.; Conti, G.O.; Ferrante, M. Trace elements in seafood from the Mediterranean Sea: An exposure risk assessment. Food Chem. Toxicol. 2018, 115, 13–19. [Google Scholar] [CrossRef]
- Ferrante, M.; Pappalardo, A.M.; Ferrito, V.; Pulvirenti, V.; Fruciano, C.; Grasso, A.; Sciacca, S.; Tigano, C.; Copat, C. Bioaccumulation of metals and biomarkers of environmental stress in Parablennius sanguinolentus (Pallas, 1814) sampled along the Italian coast (2017). Mar. Pollut. Bull. 2017, 122, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Tigano, C.; Tomasello, B.; Pulvirenti, V.; Ferrito, V.; Copat, C.; Carpinteri, G.; Mollica, E.; Sciacca, S.; Renis, M. Assessment of environmental stress in Parablennius sanguinolentus (Pallas, 1814) of the Sicilian Ionian coast. Ecotox. Environ. Saf. 2009, 74, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Brundo, M.V.; Arena, G.; Grasso, A.; Oliveri Conti, G.; Ledda, C.; Fallico, F.; Sciacca, S.; Ferrante, M. Seasonal variation of bioaccumulation in Engraulis encrasicolus (Linneaus, 1758) and related biomarkers of exposure. Ecotoxicol. Environ. Saf. 2012, 86, 31–37. [Google Scholar] [CrossRef]
- Salvaggio, A.; Tiralongo, F.; Krasakopoulou, E.; Marmara, D.; Giovos, I.; Crupi, R.; Messina, G.; Lombardo, B.M.; Marzullo, A.; Pecoraro, R.; et al. Biomarkers of Exposure to Chemical Contamination in the Commercial Fish Species Lepidopus caudatus (Euphrasen, 1788): A Particular Focus on Plastic Additives. Front. Physiol. 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brundo, M.V.; Pecoraro, R.; Marino, F.; Salvaggio, A.; Tibullo, D.; Saccone, S.; Bramanti, V.; Buccheri, M.A.; Impellizzeri, G.; Scuderi, V.; et al. Toxicity Evaluation of New Engineered Nanomaterials in Zebrafish. Front. Physiol. 2016, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, G.; Vitiello, G.; De Tommaso, G.; Abdel-Gaward, F.K.; Brundo, M.V.; Ferrante, M.; De Maio, A.; Trocchia, S.; Bianchi, A.R.; Ciarcia, G.; et al. Electron Spin Resonance (ESR) for the study of Reactive Oxygen Species (ROS) on the isolated frog skin (Pelophylax bergeri): A non-invasive method for environmental monitoring. Environ. Res. 2018, 165, 11–18. [Google Scholar] [CrossRef]
- Guerriero, G.; Brundo, M.V.; Labar, S.; Bianchi, A.R.; Trocchia, S.; Rabbito, D.; Palumbo, G.; Abdel-Gawad, F.K.; De Maio, A. Frog (Pelophylax bergeri, Gunther 1986) endocrine disruption assessment: Characterization and role of skin poly (ADp-ribose) polymerases. Environ. Sci. Pollut. Res. Int. 2018, 25, 18303–18313. [Google Scholar] [CrossRef]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef]
- Elnabris, K.J.; Muzyed, S.K.; El-Ashgar, N.M. Heavy metal concentrations in some commercially important fishes and their contribution to heavy metals exposure in Palestinian people of Gaza Strip (Palestine). J. Assoc. Arab Univ. Basic Appl. Sci. 2013, 13, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Gbogbo, F.; Arthur-Yartel, A.; Bondzie, J.A.; Dorleku, W.P.; Dadzie, S.; Kwansa-Bentum, B.; Ewool, J.; Billah, M.K.; Lamtey, A.M. Risk of heavy metal ingestion from the consumption of two commercially valuable species of fish from the fresh and coastal waters of Ghana. PLoS ONE 2018, 13, e0194682. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Li, X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef] [PubMed]
- EFSA. EFSA provides risk assessment on mercury fish: Precautionary advice given to vulnerable groups. EFSA J. 2004, 34, 1–14. [Google Scholar]
- McCarthy, F.; Shugart, L.R. Biomarkers of Environmental Contamination; McCarthy, J.F., Shugart, L.R., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1990. [Google Scholar]
- Depledge, M.; Fossi, M.C. The role of biomarker in environmental assessment: Invertebrates. Ecotoxicology 1994, 3, 173–179. [Google Scholar] [CrossRef]
- Viana, A.P.; Lucena Frédou, F. Ichthyofauna as bioindicator of environmental quality in an industrial district in the amazon estuary, Brazil. Braz. J. Biol. 2014, 74, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Tissue | Sex | Statistics | As | Cd | Co | Cr | Cu | Pb | Hg | Mn | Ni | V | Se | Sb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gill | Males | Min. | 1.115 | 0.005 | 0.465 | 2.468 | 4.746 | 2.459 | 0.030 | 78.25 | 1.452 | 4.598 | 0.246 | 0.021 | 16.43 |
| Max. | 1.924 | 0.010 | 0.513 | 3.215 | 5.483 | 5.896 | 0.058 | 130.5 | 3.145 | 9.452 | 0.495 | 0.059 | 19.75 | ||
| Mean | 1.536 | 0.007 | 0.496 | 2.851 | 5.137 | 3.706 | 0.043 | 104.7 | 2.408 | 6.501 | 0.376 | 0.034 | 18.76 | ||
| S.D. | 0.234 | 0.002 | 0.017 | 0.183 | 0.260 | 1.032 | 0.009 | 18.12 | 0.559 | 1.400 | 0.074 | 0.012 | 1.094 | ||
| Females | Min. | 1.248 | 0.022 | 0.745 | 2.853 | 3.145 | 5.424 | 0.021 | 78.25 | 2.139 | 6.300 | 0.156 | 0.021 | 8.120 | |
| Max. | 1.570 | 0.037 | 0.985 | 3.851 | 4.926 | 7.952 | 0.040 | 120.3 | 2.770 | 9.485 | 0.235 | 0.062 | 12.37 | ||
| Mean | 1.429 | 0.029 | 0.880 | 3.354 | 4.163 | 6.486 | 0.032 | 97.80 | 2.517 | 8.045 | 0.189 | 0.044 | 10.11 | ||
| S.D. | 0.106 | 0.005 | 0.079 | 0.306 | 0.483 | 0.735 | 0.006 | 11.53 | 0.200 | 1.253 | 0.024 | 0.013 | 1.394 | ||
| Gastrointestnal content | Males | Min. | 0.302 | 0.001 | 0.034 | 0.345 | 1.952 | 0.135 | 0.174 | 1.324 | 0.041 | 0.180 | 0.141 | <0.020 | 8.125 |
| Max. | 0.601 | 0.007 | 0.064 | 0.699 | 2.770 | 0.195 | 0.251 | 1.520 | 0.095 | 0.264 | 0.195 | <0.020 | 12.45 | ||
| Mean | 0.405 | 0.003 | 0.048 | 0.544 | 2.358 | 0.165 | 0.197 | 1.390 | 0.066 | 0.220 | 0.177 | <0.020 | 9.873 | ||
| S.D. | 0.084 | 0.002 | 0.009 | 0.107 | 0.270 | 0.020 | 0.023 | 0.053 | 0.018 | 0.028 | 0.016 | / | 1.122 | ||
| Females | Min. | 0.421 | 0.001 | 0.021 | 0.485 | 1.214 | 0.214 | 0.182 | 1.100 | 0.065 | 0.215 | 0.234 | <0.020 | 8.420 | |
| Max. | 0.621 | 0.009 | 0.064 | 0.741 | 3.254 | 0.512 | 0.260 | 2.164 | 0.164 | 0.354 | 0.485 | <0.020 | 12.48 | ||
| Mean | 0.505 | 0.005 | 0.037 | 0.620 | 2.092 | 0.324 | 0.210 | 1.903 | 0.099 | 0.280 | 0.357 | <0.020 | 10.88 | ||
| S.D. | 0.067 | 0.003 | 0.011 | 0.095 | 0.585 | 0.088 | 0.027 | 0.348 | 0.029 | 0.049 | 0.087 | / | 1.331 | ||
| Muscle | Males | Min. | 0.111 | 0.001 | <0.008 | 0.325 | 0.218 | 0.007 | 0.005 | 0.230 | <0.007 | <0.025 | 0.075 | <0.020 | 0.796 |
| Max. | 0.194 | 0.009 | <0.008 | 0.852 | 0.324 | 0.021 | 0.016 | 0.465 | <0.007 | <0.025 | 0.164 | <0.020 | 1.500 | ||
| Mean | 0.151 | 0.004 | <0.008 | 0.522 | 0.272 | 0.014 | 0.009 | 0.363 | <0.007 | <0.025 | 0.111 | <0.020 | 1.101 | ||
| S.D. | 0.033 | 0.002 | / | 0.146 | 0.041 | 0.005 | 0.003 | 0.072 | / | / | 0.023 | / | 0.213 | ||
| Females | Min. | 0.114 | 0.002 | <0.008 | 0.596 | 0.222 | 0.001 | 0.005 | 1.108 | 0.011 | <0.025 | 0.085 | <0.020 | 1.745 | |
| Max. | 0.384 | 0.006 | <0.008 | 0.771 | 0.513 | 0.009 | 0.014 | 1.345 | 0.035 | <0.025 | 0.254 | <0.020 | 2.224 | ||
| Mean | 0.212 | 0.004 | <0.008 | 0.692 | 0.341 | 0.003 | 0.010 | 1.201 | 0.021 | <0.025 | 0.137 | <0.020 | 2.072 | ||
| S.D. | 0.086 | 0.001 | / | 0.055 | 0.079 | 0.002 | 0.003 | 0.078 | 0.008 | / | 0.057 | / | 0.141 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvaggio, A.; Pecoraro, R.; Copat, C.; Ferrante, M.; Grasso, A.; Scalisi, E.M.; Ignoto, S.; Bonaccorsi, V.S.; Messina, G.; Lombardo, B.M.; et al. Bioaccumulation of Metals/Metalloids and Histological and Immunohistochemical Changes in the Tissue of the European Hake, Merluccius merluccius (Linnaeus, 1758) (Pisces: Gadiformes: Merlucciidae), for Environmental Pollution Assessment . J. Mar. Sci. Eng. 2020, 8, 712. https://doi.org/10.3390/jmse8090712
Salvaggio A, Pecoraro R, Copat C, Ferrante M, Grasso A, Scalisi EM, Ignoto S, Bonaccorsi VS, Messina G, Lombardo BM, et al. Bioaccumulation of Metals/Metalloids and Histological and Immunohistochemical Changes in the Tissue of the European Hake, Merluccius merluccius (Linnaeus, 1758) (Pisces: Gadiformes: Merlucciidae), for Environmental Pollution Assessment . Journal of Marine Science and Engineering. 2020; 8(9):712. https://doi.org/10.3390/jmse8090712
Chicago/Turabian StyleSalvaggio, Antonio, Roberta Pecoraro, Chiara Copat, Margherita Ferrante, Alfina Grasso, Elena Maria Scalisi, Sara Ignoto, Vincenza Serena Bonaccorsi, Giuseppina Messina, Bianca Maria Lombardo, and et al. 2020. "Bioaccumulation of Metals/Metalloids and Histological and Immunohistochemical Changes in the Tissue of the European Hake, Merluccius merluccius (Linnaeus, 1758) (Pisces: Gadiformes: Merlucciidae), for Environmental Pollution Assessment " Journal of Marine Science and Engineering 8, no. 9: 712. https://doi.org/10.3390/jmse8090712