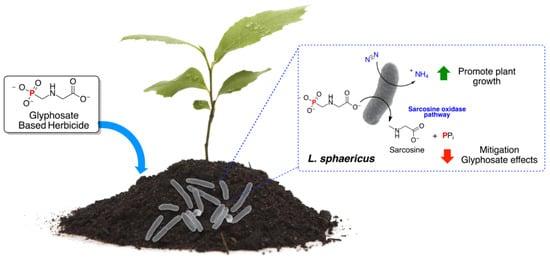
Glyphosate Bioremediation through the Sarcosine Oxidase Pathway Mediated by Lysinibacillus sphaericus in Soils Cultivated with Potatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crop Experimental Design
2.2. L. sphaericus Strains and Inoculum Preparation
2.3. Parcels Treatment Application
2.4. Nitrate and Ammonium Determination
2.5. Glyphosate and AMPA Determination by Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry (UHPLC–MS)
2.6. Glyphosate Determination by Ninhydrin Reaction
2.6.1. Extraction of Glyphosate from the Soil and Standard Addition Method
2.6.2. Ninhydrin Derivatization
2.6.3. Quantification by Standard Addition
2.7. DNA Extraction and Sarcosine Oxidase Gene Presence
2.8. Follicular Area Image Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Nitrate and Ammonium Concentrations
3.2. Glyphosate Degradation Quantified by UHPLC-MS/M
3.3. Glyphosate Determination by the Ninhydrin Reaction
3.4. Sarcosine Oxidase Gene Presence
3.5. Addition, Mitigation, and Nitrogen Fixation Activity of L. sphaericus in the Potato Crop
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouided, B.; Abderrahmane, B. Isolation and characterization of glyphosate-degrading bacteria from different soils of Algeria. Afr. J. Microbiol. Res. 2016, 7, 5587–5595. [Google Scholar] [CrossRef]
- Glycine, W.; Stizolobium, E.; De Glyphosate, B.; Glycine, R. De Biodegradation of Glyphosate in Rhizospheric Soil Cultivated with Glycine max, Canavalia ensiformis and Stizolobium aterrimum. Planta Daninha 2009, 781–787. [Google Scholar]
- Funke, T.; Healy-Fried, M.L.; Han, H.; Alberg, D.G.; Bartlett, P.A.; Schönbrunn, E. Differential inhibition of class I and class II 5-enolpyruvylshikimate-3- phosphate synthases by tetrahedral reaction intermediate analogues. Biochemistry 2007, 46, 13344–13351. [Google Scholar] [CrossRef] [PubMed]
- Sutton, K.A.; Breen, J.; Russo, T.A.; Schultz, L.W.; Umland, T.C. Crystal structure of 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase from the ESKAPE pathogen Acinetobacter baumannii. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016, 72, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xia, H.; Yang, X.; Xu, T.; Si, H.J.; Cai, X.X.; Wang, F.; Su, J.; Snow, A.A.; Lu, B.R. A novel 5-enolpyruvoylshikimate-3-phosphate (EPSP) synthase transgene for glyphosate resistance stimulates growth and fecundity in weedy rice (Oryza sativa) without herbicide. New Phytol. 2014, 202, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Cuhra, M.; Bøhn, T.; Cuhra, P. Glyphosate: Too Much of a Good Thing? Front. Environ. Sci. 2016, 4, 1–14. [Google Scholar] [CrossRef]
- Gaupp-Berghausen, M.; Hofer, M.; Rewald, B.; Zaller, J.G. Glyphosate-based herbicides reduce the activity and reproduction of earthworms and lead to increased soil nutrient concentrations. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Blumberg, B.; Antoniou, M.N.; Benbrook, C.M.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; et al. Is it time to reassess current safety standards for glyphosate-based herbicides? J. Epidemiol. Community Health 2017, 71, 613–618. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Monograph Working Group, IARC, Lyon, France. Evaluation of five organophosphate insecticides and herbicides. In Some Organophosphate Insecticides and Herbicides; IARC: Lyon, France, 2015; ISBN 978-92-832-0178-6. [Google Scholar]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Cressey, D. Widely used herbicide linked to cancer. Nature 2015. [Google Scholar] [CrossRef]
- Sviridov, A.V.; Shushkova, T.V.; Ermakova, I.T.; Ivanova, E.V.; Epiktetov, D.O.; Leontievsky, A.A. Microbial degradation of glyphosate herbicides (Review). Appl. Biochem. Microbiol. 2015, 51, 188–195. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef] [PubMed]
- la Cecilia, D.; Maggi, F. Analysis of glyphosate degradation in a soil microcosm. Environ. Pollut. 2018, 233, 201–207. [Google Scholar] [CrossRef] [PubMed]
- González-Valenzuela, L.E.; Dussán, J. Molecular assessment of glyphosate-degradation pathway via sarcosine intermediate in Lysinibacillus sphaericus. Environ. Sci. Pollut. Res. 2018, 25, 22790–22796. [Google Scholar] [CrossRef] [PubMed]
- Hove-Jensen, B.; Zechel, D.L.; Jochimsen, B. Utilization of glyphosate as phosphate source: Biochemistry and genetics of bacterial carbon-phosphorus lyase. Microbiol. Mol. Biol. Rev. 2014, 78, 176–197. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015, 16, 490–491. [Google Scholar] [CrossRef]
- Gómez-Garzón, C.; Hernández-Santana, A.; Dussán, J. Comparative genomics reveals Lysinibacillus sphaericus group comprises a novel species. BMC Genom. 2016, 17, 709. [Google Scholar] [CrossRef]
- Martínez, S.A.; Dussán, J. Lysinibacillus sphaericus plant growth promoter bacteria and lead phytoremediation enhancer with Canavalia ensiformis. Environ. Prog. Sustain. Energy 2018, 37, 276–282. [Google Scholar] [CrossRef]
- Aguirre-Monroy, A.M.; Santana-Martínez, J.C.; Dussán, J. Lysinibacillus sphaericus as a Nutrient Enhancer during Fire-Impacted Soil Replantation. Appl. Environ. Soil Sci. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Hernández-Santana, A.; Dussán, J. Lysinibacillus sphaericus proved to have potential for the remediation of petroleum hydrocarbons. Soil Sediment Contam. 2018, 27, 538–549. [Google Scholar] [CrossRef]
- Peña-Montenegro, T.D.; Dussán, J. Genome sequence and description of the heavy metal tolerant bacterium Lysinibacillus sphaericus strain OT4b.31. Stand. Genomic Sci. 2013, 9, 42–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, L.C.; Dussán, J. Synergistic Activity Between S-Layer Protein and Spore–Crystal Preparations from Lysinibacillus sphaericus Against Culex quinquefasciatus Larvae. Curr. Microbiol. 2017, 74, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Garzón, C.; Hernández-Santana, A.; Dussán, J. A genome-scale metabolic reconstruction of Lysinibacillus sphaericus unveils unexploited biotechnological potentials. PLoS ONE 2017, 12, e0179666. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.; Silva-Quintero, L.; Dussán, J. Complete genome sequencing and comparative genomic analysis of functionally diverse Lysinibacillus sphaericus III(3)7. Genom. Data 2016, 9, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas-Pinzón, P.A.; Dussán, J. Efficacy of the vegetative cells of Lysinibacillus sphaericus for biological control of insecticide-resistant Aedes aegypti. Parasit. Vectors 2017, 10, 231. [Google Scholar] [CrossRef]
- Hernández-Santana, A.; Gómez-Garzón, C.; Dussán, J. Complete Genome Sequence of Lysinibacillus sphaericus WHO Reference Strain 2362. Genome Announc. 2016, 4, e00545-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Potato Center. Why Are Potatoes Important? International Potato Center: Lima, Perú, 2017. [Google Scholar]
- Spooner, D.M.; Núñez, J.; Trujillo, G.; Herrera, M.d.R.; Guzmán, F.; Ghislain, M. Extensive simple sequence repeat genotyping of potato landraces supports a major reevaluation of their gene pool structure and classification. Proc. Natl. Acad. Sci. USA 2007, 104, 19398–19403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghislain, M.; Andrade, D.; Rodríguez, F.; Hijmans, R.J.; Spooner, D.M. Genetic analysis of the cultivated potato Solanum tuberosum L. Phureja Group using RAPDs and nuclear SSRs. Theor. Appl. Genet. 2006, 113, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Spooner, D.M. The potato: Evolution, biodiversity and genetic resources. J.G. Hawkes. Am. Potato J. 1990, 67, 733–735. [Google Scholar] [CrossRef]
- Sun, L.; Kong, D.; Gu, W.; Guo, X.; Tao, W.; Shan, Z.; Wang, Y.; Wang, N. Determination of glyphosate in soil/sludge by high performance liquid chromatography. J. Chromatogr. A 2017, 1502, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Saxberg, B.E.H.; Kowalski, B.R. Generalized Standard Addition Method. Anal. Chem. 1979, 51, 1031–1038. [Google Scholar] [CrossRef]
- Bhattacharjee, R.B.; Singh, A.; Mukhopadhyay, S.N. Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: Prospects and challenges. Appl. Microbiol. Biotechnol. 2008, 80, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Borggaard, O.K.; Duke, S.O.; Powles, S.B. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: Areview. Pest Manag. Sci. 2008, 63, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, P.; Bhaskara, B.L. Direct Sensitive spectrophotometric assessment of carbofuran using dapsone as a new chromogenic reagent in formulations and environmental samples. Eclet. Quim. 2006, 31, 43–48. [Google Scholar] [CrossRef]
- Friedman, M. Applications of the Ninhydrin Reaction for Analysis of Amino Acids, Peptides, and Proteins to Agricultural and Biomedical Sciences. J. Agric. Food Chem. 2004, 52, 385–406. [Google Scholar] [CrossRef]
- Moneke, A.; Okpala, G.; Anyanwu, C. Biodegradation of glyphosate herbicide in vitro using bacterial isolates from four rice fields. Afr. J. Biotechnol. 2010, 9, 4067–4074. [Google Scholar]
- Arfarita, N.; Imai, T.; Kanno, A.; Yarimizu, T.; Xiaofeng, S.; Jie, W.; Higuchi, T.; Akada, R. The potential use of trichoderma viride strain FRP3 in biodegradation of the herbicide glyphosate. Biotechnol. Biotechnol. Equip. 2013, 27, 3518–3521. [Google Scholar] [CrossRef]
- Cheloufi, R.; Messaadia, H.; Alayat, H. Biodegradation of herbicides by Pseudomonas aeruginosa in two soils types of the Bou Namoussa irrigable perimeter (Algerian Extreme Northeast): Effects on mineral nutrition (P2O5 and NO3−). J. Mater. Environ. Sci. 2017, 8, 2513–2521. [Google Scholar]
- Rivera, S.; Dussán, J. Trial evaluation of toxicity and pathology in murine tissues using Bacillus sphaericus Colombian strain. Actual Biol. 2008, 30, 125–134. [Google Scholar]











| Treatment | B | G | BG | C |
|---|---|---|---|---|
| Description | Bacterial mixture containing five strains of Lysinibacillus sphaericus. | 1.69 mg/mL glyphosate aqueous solution. | Bacterial mixture containing five strains of L. sphaericus and 1.69 mg/mL glyphosate solution. | Water from the same source used in the other treatments. |
| Strain | Source |
|---|---|
| CBAM5 | Hydrocarbon-contaminated soil [23]. |
| III (3) 7 | Oak forest soil [26]. |
| OT.4b.49 | Native Colombian strain [19]. |
| OT.4b.31 | Native Colombian strain [23]. |
| 2362 | WHO entomopathogenic reference strain [28]. |
| BLAST Result | Accession | Genome Region | B | BG | ||
|---|---|---|---|---|---|---|
| Identity (%) | Query Cover | Identity (%) | Query Cover | |||
| L. sphaericus strain 2362, complete genome. | CP015224.1 | 3365804–3365863 | 98.33 | 83% | 95.38 | 89% |
| L. sphaericus III(3)7, complete genome. | CP014856.1 | 264109–264168 | 98.33 | 83% | 95.38 | 89% |
| L. sphaericus strain OT4b.25, complete genome. | CP014643.1 | 1131747–1131688 | 98.33 | 83% | 92.96 | 98% |
| L. sphaericus C3-41, complete genome. | CP000817.1 | 117009–116950 | 98.33 | 83% | 92.96 | 98% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Rodríguez, M.; Melo, C.; Jiménez, E.; Dussán, J. Glyphosate Bioremediation through the Sarcosine Oxidase Pathway Mediated by Lysinibacillus sphaericus in Soils Cultivated with Potatoes. Agriculture 2019, 9, 217. https://doi.org/10.3390/agriculture9100217
Pérez Rodríguez M, Melo C, Jiménez E, Dussán J. Glyphosate Bioremediation through the Sarcosine Oxidase Pathway Mediated by Lysinibacillus sphaericus in Soils Cultivated with Potatoes. Agriculture. 2019; 9(10):217. https://doi.org/10.3390/agriculture9100217
Chicago/Turabian StylePérez Rodríguez, Mario, Carol Melo, Elizabeth Jiménez, and Jenny Dussán. 2019. "Glyphosate Bioremediation through the Sarcosine Oxidase Pathway Mediated by Lysinibacillus sphaericus in Soils Cultivated with Potatoes" Agriculture 9, no. 10: 217. https://doi.org/10.3390/agriculture9100217