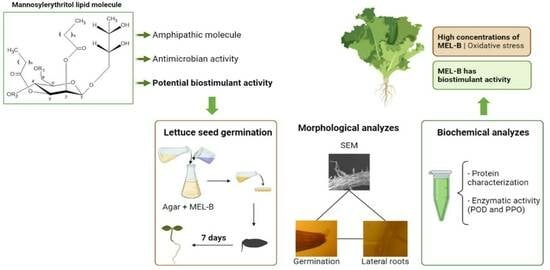
The Biosurfactants Mannosylerythritol Lipids (MELs) as Stimulant on the Germination of Lactuca sativa L.
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.1.1. Growing Medium Containing MEL-B for Lettuce Seed Germination
2.1.2. Contact Angle and Surface Tension
2.2. Germination Test
2.2.1. Germination Speed Index (GSI)
2.2.2. Mean Germination Time (MGT)
2.3. Morphological Parameters in Lettuce Cultivation
2.4. Physicochemical Characterizations of Total Proteins and Activity of Peroxidase and Polyphenol Oxidase Enzymes
Crude Enzymatic Extraction
2.5. Protein Content
2.5.1. Peroxidase Activity
2.5.2. Polyphenoloxidase Activity
2.6. Statistical Analysis
3. Results
3.1. Contact Angle and Surface Tension
3.2. Germination Properties
3.3. Morphology of the Roots
3.4. Quantification of Protein and Enzyme Activity
4. Discussion
4.1. Interpretation of the Behavior of the Contact Angle and Surface Tension
4.2. Assessing Germination Properties
4.3. Morphological Changes of Roots
4.4. Biochemical Analyzes after Treatment with MEL-B
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, K.; Bach, M.; Hartmann, H.; Spiteller, M.; Frede, H.-G. Point- and Nonpoint-Source Pesticide Contamination in the Zwester Ohm Catchment, Germany. J. Environ. Qual. 2002, 31, 309. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Johnston, C.T.; Teppen, B.J.; Boyd, S.A. Potential contributions of smectite clays and organic matter to pesticide retention in soils. J. Agric. Food Chem. 2001, 49, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.V.A.; de Albuquerque, G.S.C. Agrotóxicos e seus impactos na saúde humana e ambiental: Uma revisão sistemática. Saúde Em Debate 2018, 42, 518–534. [Google Scholar] [CrossRef]
- de Moraes, R.F. Agrotóxicos no Brasil: Padrões de Uso, Político da Regulação e Prevenção da Captura Regulatória; Instituto de Pesquisa Econômica Aplicada (IPEA): Brasília, Brazil, 2019; 84p. [Google Scholar]
- Aenda, Associação Brasileira dos Defensivos Genéricos, Prod. Regist. No Bras. (2019). Available online: https://www.aenda.org.br/biologicos/ (accessed on 11 March 2023).
- European Union Regulations, EU Regulation of the European Parliament and of the Council Laying Down Rules on Making EU Fertilizer Products Available on the Market and Amending Regulations (EC) no. 1069/2009 and (EC) no. 1107/2009 and Repealing the Regulation (EC) No. 2003/2003. 2, (2019). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 11 March 2023).
- EMBRAPA, Biostimulants and Production of Vegetables. 2016. Available online: https://urlshortner.org/LGurU (accessed on 11 March 2023).
- Buxdorf, K.; Rahat, I.; Gafni, A.; Levy, M. The epiphytic fungus Pseudozyma aphidis induces jasmonic acid-and salicylic acid/nonexpressor of PR1-independent local and systemic resistance. Plant Physiol. 2013, 161, 2014–2022. [Google Scholar] [CrossRef]
- Yan, F.; Xu, S.; Chen, Y.; Zheng, X. Effect of rhamnolipids on Rhodotorula glutinis biocontrol of Alternaria alternata infection in cherry tomato fruit. Postharvest Biol. Technol. 2014, 97, 32–35. [Google Scholar] [CrossRef]
- Sieverding, E. Increasing Yelds by the Use of Sophorolipids. Patent EP3142490A1, 22 March 2017. [Google Scholar]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Characterisation, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP0995 for application in the petroleum industry. Colloids Surf. B Biointerfaces 2013, 102, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.D.; de Luna, J.M.; de Campos Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014, 17, 34–38. [Google Scholar] [CrossRef]
- Kim, H.-S.; Jeon, J.-W.; Kim, B.-H.; Ahn, C.-Y.; Oh, H.-M.; Yoon, B.-D. Extracellular production of a glycolipid biosurfactant, mannosylerythritol lipid, by Candida sp. SY16 using fed-batch fermentation. Appl. Microbiol. Biotechnol. 2006, 70, 391–396. [Google Scholar] [CrossRef]
- Fukuoka, T.; Morita, T.; Konishi, M.; Imura, T.; Kitamoto, D. Characterization of new glycolipid biosurfactants, tri-acylated mannosylerythritol lipids, produced by Pseudozyma yeasts. Biotechnol. Lett. 2007, 29, 1111–1118. [Google Scholar] [CrossRef]
- Günther, M.; Grumaz, C.; Lorenz, S.; Stevens, P.; Lindemann, E.; Hirth, T.; Sohn, K.; Zibek, S.; Rupp, S. The transcriptomic profile of Pseudozyma aphidis during production of mannosylerythritol lipids. Appl. Microbiol. Biotechnol. 2015, 99, 1375–1388. [Google Scholar] [CrossRef]
- De Andrade, C.J.; de Andrade, L.M.; Rocco, S.A.; Sforça, M.L.; Pastore, G.M.; Jauregi, P. A novel approach for the production and purification of mannosylerythritol lipids (MEL) by Pseudozyma tsukubaensis using cassava wastewater as substrate. Sep. Purif. Technol. 2017, 180, 157–167. [Google Scholar] [CrossRef]
- Matosinhos, R.D.; Cesca, K.; Carciofi, B.A.M.; de Oliveira, D.; de Andrade, C.J. Mannosylerythritol lipids as green pesticides and plant biostimulants. J. Sci. Food Agric. 2023, 103, 37–47. [Google Scholar] [CrossRef]
- Ceresa, C.; Fracchia, L.; Fedeli, E.; Porta, C. Recent Advances in Biomedical, Therapeutic and Pharmaceutical Applications of Microbial Surfactants. Pharmaceutics 2021, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Karamchandani, B.M. Chitosan and its derivatives: Promising biomaterial in averting fungal diseases of sugarcane and other crops. J. Basic Microbiol. 2022, 62, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Aranda, F.J.; Teruel, J.A.; Espuny, M.J.; Marqués, A.; Manresa, Á.; Ortiz, A. Permeabilization of biological and artificial membranes by a bacterial dirhamnolipid produced by Pseudomonas aeruginosa. J. Colloid Interface Sci. 2010, 341, 240–247. [Google Scholar] [CrossRef]
- Karamchandani, B.M.; Maurya, P.A.; Dalvi, S.G.; Waghmode, S. Synergistic Activity of Rhamnolipid Biosurfactant and Nanoparticles Synthesized Using Fungal Origin Chitosan Against Phytopathogens. Front. Bioeng. Biotchnol. 2022, 10, 917105. [Google Scholar] [CrossRef]
- Chopra, A.; Kumar, U.; Rahi, P.; Satpute, S. Biocatalysis and Agricultural Biotechnology Plant growth promoting potential of Brevibacterium sediminis A6 isolated from the tea rhizosphere of Assam, India. Biocatal. Agric. Biotechnol. 2020, 27, 101610. [Google Scholar] [CrossRef]
- Handore, A.V.; Khandelwal, S.R.; Karmakar, R.; Gupta, D.L.; Jagtap, V.S. Applications and future prospects of biosurfactants. In Microbial Surfactants; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Lima, J.E.S.; Fernandes, S.V. Determinação do Ângulo de Contato, Tensão Superficial e Trabalho de Adesão de um Primer Adesivo Uretânico; Centro Universitário ENIAC: Guarulhos, Brazil, 2019; pp. 1–6. [Google Scholar]
- Brasil. Regras Para Análise de Sementes (RAS). 2009. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf (accessed on 26 March 2023).
- Maguire, J.D. Speed of germination: Aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Labouriau, L.G. A germinação das Sementes; Secretaria Geral da OEA: Washington, DC, USA, 1983; 174p. [Google Scholar]
- Zhang, N.; Hasenstein, K.H. Initiation and elongation of lateral roots in Lactuca sativa. Int. J. Plant Sci. 1999, 160, 511–519. [Google Scholar] [CrossRef]
- Luiz, V.; Lovaglio, R.B.; Tozzi, H.H.; Contiero, J. Rhamnolipids: A New Application in Seeds Development. J. Med. Biol. Sci. Res. 2015, 1, 100–106. [Google Scholar]
- Al Shehadat, S.; Gorduysus, M.O.; Hamid, S.S.; Abdullah, N.A.; Samsudin, A.R.; Ahmad, A. Optimization of scanning electron microscope technique for amniotic membrane investigation: A preliminary study. Nature 2018, 388, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pomory, C.M. Color development time of the Lowry protein assay. Anal. Biochem. 2008, 378, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fang, W.; Lu, H.; Zhu, R.; Lu, L.; Zheng, X.; Yu, T. Inhibition of green mold disease in mandarins by preventive applications of methyl jasmonate and antagonistic yeast Cryptococcus laurentii. Postharvest Biol. Technol. 2014, 88, 72–78. [Google Scholar] [CrossRef]
- Matsuno, H.; Uritani, I. Physiological behavior of peroxidase isozymes in sweet potato root tissue injured by cutting or with black rot. Plant Cell Physiol. 1972, 13, 1091–1101. [Google Scholar] [CrossRef]
- Ördög, V.; Zoltán, M. Water and nutrients in plants. Plant Physiol. 2011, 2–32. Available online: http://www.tankonyvtar.hu/en/tartalom/tamop425/0010_1A_Book_angol_01_novenyelettan/ch02.html#id466693 (accessed on 5 February 2023).
- Luciano dos Reis, V. Extratos Vegetais no Controle de Fungos Fitopatogênicos à Soja; Federal University of Grande Dourados: Dourados, Brazil, 2009; pp. 1–99. [Google Scholar]
- Adamson, A.; Gast, A. Physical Chemistry of Surfaces; John Wiley & Sons, Inc.: Toronto, ON, Canada, 1997. [Google Scholar]
- Schultz, J.; Rosado, A.S. Extreme environments: A source of biosurfactants for biotechnological applications. Extremophiles 2020, 24, 189–206. [Google Scholar] [CrossRef]
- Sancheti, A.; Ju, L.K. Rhamnolipid Effects on Water Imbibition, Germination, and Initial Root and Shoot Growth of Soybeans. J. Surfactants Deterg. 2020, 23, 371–381. [Google Scholar] [CrossRef]
- Karthika, S.; Midhun, S.J.; Jisha, M.S. A potential antifungal and growth-promoting bacterium Bacillus sp. KTMA4 from tomato rhizosphere. Microb. Pathog. 2020, 142, 104049. [Google Scholar] [CrossRef]
- Parr, J.F.; Norman, A.G. Norman, Considerations in the Use of Surfactants in Plant Systems: A Review. Bot. Gaz. 1965, 126, 86–96. [Google Scholar] [CrossRef]
- Poštić, D.; Štrbanović, R.; Tabaković, M.; Popović, T.; Ćirić, A.; Banjac, N.; Trkulja, N.; Stanisavljević, R. Germination and the initial seedling growth of lettuce, celeriac and wheat cultivars after micronutrient and a biological application pre-sowing seed treatment. Plants 2021, 10, 1913. [Google Scholar] [CrossRef] [PubMed]
- Sieverding, E. (12) Patent Application Publication (10). U.S. Patent 2017/0094968 A1, 6 April 2017. [Google Scholar]
- Khare, E.; Kumar, N. Biosurfactant based formulation of Pseudomonas guariconensis LE3 with multifarious plant growth promoting traits controls charcoal rot disease in Helianthus annus. World J. Microbiol. Biotechnol. 2021, 37, 55. [Google Scholar] [CrossRef] [PubMed]
- Tikoria, R.; Kaur, A.; Ohri, P. Plant Physiology and Biochemistry Physiological, biochemical and structural changes in tomato plants by vermicompost application in different exposure periods under glass house conditions. Plant Physiol. Biochem. 2023, 197, 107656. [Google Scholar] [CrossRef] [PubMed]
- Diehl, B.G. Preparation and Characterization of Lignin-Protein Covalent Linkages. Ph.D. Thesis, The Pennsylvania State University, State College, PA, USA, 2014. Available online: http://findtext.lib.ncsu.edu/?ctx_ver=Z39.88-2004&ctx_enc=info:ofi/enc:UTF-8&rfr_id=info:sid/ProQuest+Dissertations+%2526+Theses+Full+Text&rft_val_fmt=info:ofi/fmt:kev:mtx:dissertation&rft.genre=dissertations+%2526+theses&rft.jtitle=&rft.atitle=&rft.au=Di (accessed on 26 March 2023).






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matosinhos, R.D.; Cesca, K.; Carciofi, B.A.M.; de Oliveira, D.; de Andrade, C.J. The Biosurfactants Mannosylerythritol Lipids (MELs) as Stimulant on the Germination of Lactuca sativa L. Agriculture 2023, 13, 1646. https://doi.org/10.3390/agriculture13091646
Matosinhos RD, Cesca K, Carciofi BAM, de Oliveira D, de Andrade CJ. The Biosurfactants Mannosylerythritol Lipids (MELs) as Stimulant on the Germination of Lactuca sativa L. Agriculture. 2023; 13(9):1646. https://doi.org/10.3390/agriculture13091646
Chicago/Turabian StyleMatosinhos, Renato Dias, Karina Cesca, Bruno Augusto Mattar Carciofi, Débora de Oliveira, and Cristiano José de Andrade. 2023. "The Biosurfactants Mannosylerythritol Lipids (MELs) as Stimulant on the Germination of Lactuca sativa L." Agriculture 13, no. 9: 1646. https://doi.org/10.3390/agriculture13091646