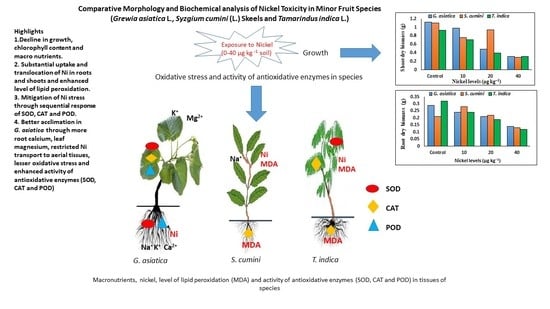
Comparative Morphology and Biochemical Analysis of Nickel Toxicity in Minor Fruit Species (Grewia asiatica L., Syzgium cumini (L.) Skeels and Tamarindus indica L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Nickel Treatments
2.2. Growth Components
2.3. Photosynthetic Pigments
2.4. Determination of Macronutrients and Nickel
2.5. MDA in Tissues
2.6. Activity of Antioxidant Enzymes
2.7. Statistical Analysis
3. Results
3.1. Modulation of Growth Components
3.2. Photosynthetic Apparatus
3.3. MDA Production and Nickel Uptake in Tissues
3.4. Nutritional Status of Species for Macronutrients
3.5. Antioxidant Enzymes (SOD, CAT and POD)
4. Discussion
4.1. Response of Growth and Photosynthesis
4.2. ROS Production and Antioxidant Activity
4.3. Nutrient Uptake and Ni Toxicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meshram, P.; Pandey, B.D. Advanced Review on Extraction of Nickel from Primary and Secondary Sources. Miner. Process. Extr. Met. Rev. 2018, 40, 157–193. [Google Scholar] [CrossRef]
- Brown, L.R. A new era unfolds. Challenge 1993, 36, 37–46. [Google Scholar] [CrossRef]
- Iyaka, Y.A. Nickel in soils: A review of its distribution and impacts. Sci. Res. Essays 2011, 6, 6774–6777. [Google Scholar]
- Radha, T.; Mathew, L. Fruit Crops; New India Publishing: New Delhi, India, 2007; Volume 3. [Google Scholar]
- Des, U. World Economic and Social Survey 2013: Sustainable Development Challenges; United Nations, Department of Economic and Social Affairs: New York, NY, USA, 2013; pp. 123–136. [Google Scholar]
- Visioli, G.; Conti, F.D.; Gardi, C.; Menta, C. Germination and root elongation bioassays in six different plant species for testing Ni contamination in soil. Bull. Environ. Contam. Toxicol. 2014, 92, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Singh, M.P.; Kaur, S.; Bhardwaj, R.; Zheng, B.; Sharma, A. Modulation of the functional components of growth, photosynthesis, and anti-oxidant stress markers in cadmium Exposed Brassica juncea L. Plants 2019, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Zeeshan, M.; Ahmad, W.; Hussain, F.; Ahamd, W.; Numan, M.; Shah, M.; Ahmad, I. Phytostabalization of the heavy metals in the soil with biochar applications, the impact on chlorophyll, carotene, soil fertility and tomato crop yield. J. Clean. Prod. 2020, 255, 120318–120323. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Matraszek, R.; Hawrylak-Nowak, B.; Chwil, S.; Chwil, M. Macronutrient composition of nickel-treated wheat under different sulfur concentrations in the nutrient solution. Environ. Sci. Pollut. Res. 2016, 23, 5902–5914. [Google Scholar] [CrossRef] [Green Version]
- Bhalerao, S.A.; Sharma, A.S.; Poojari, A.C. Toxicity of nickel in plants. Int. J. Pure Appl. Biosci. 2015, 3, 345–355. [Google Scholar]
- Kohli, S.K.; Handa, N.; Gautam, V.; Bali, S.; Sharma, A.; Khanna, K.; Arora, S.; Thukral, A.K.; Ohri, P.; Karpets, Y.V. ROS Signaling in Plants under Heavy Metal Stress. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Singapore, 2017; pp. 185–214. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Abid, R.; Manzoor, M.; De Oliveira, L.M.; da Silva, E.; Rathinasabapathi, B.; Rensing, C.; Mahmood, S.; Liu, X.; Ma, L.Q. Interactive effects of As, Cd and Zn on their uptake and oxidative stress in As-hyperaccumulator Pteris vittata. Environ. Pollut. 2019, 248, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Abd_Allah, E.F.; Hashem, A.; Alam, P.; Ahmad, P. Silicon alleviates nickel-induced oxidative stress by regulating antioxidant defense and glyoxalase systems in mustard plants. J. Plant Growth Regul. 2019, 38, 1260–1273. [Google Scholar] [CrossRef]
- Priyanka, N.; Geetha, N.; Manish, T.; Sahi, S.; Venkatachalam, P. Zinc oxide nanocatalyst mediates cadmium and lead toxicity tolerance mechanism by differential regulation of photosynthetic machinery and antioxidant enzymes level in cotton seedlings. Toxicol. Rep. 2021, 8, 295–302. [Google Scholar]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020, 399, 123020–123031. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Ahmad, P.; Alam, P.; Balawi, T.H.; Altalayan, F.H.; Ahanger, M.A.; Ashraf, M. Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere 2020, 244, 125480–125538. [Google Scholar] [CrossRef]
- Abuelsoud, W.; Cortleven, A.; Schmülling, T. Photoperiod stress induces an oxidative burst-like response and is associated with increased apoplastic peroxidase and decreased catalase activities. J. Plant Physiol. 2020, 253, 153252–153268. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mir, M.A.; Abd-Allah, E.F.; Ahmad, P.; Gucel, S. Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front. Plant Sci. 2016, 7, 591–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlowski, T.T.; Pallardy, S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosugi, H.; Kikugawa, K. Potential thiobarbituric acid-reactive substances in peroxidized lipids. Free Radic. Biol. Med. 1989, 7, 205–208. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Ann. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Zhang, F.-Q.; Wang, Y.-S.; Lou, Z.-P.; Dong, J.-D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989; Volume 54, pp. 71–82. [Google Scholar]
- Sreekanth, T.; Nagajyothi, P.; Lee, K.; Prasad, T. Occurrence, physiological responses and toxicity of nickel in plants. Int. J. Environ. Sci. Technol. 2013, 10, 1129–1140. [Google Scholar] [CrossRef] [Green Version]
- Seregin, I.; Kozhevnikova, A. Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ. J. Plant Physiol. 2008, 55, 1–22. [Google Scholar] [CrossRef]
- Netty, S.; Wardiyati, T.; Maghfoer, M.; Handayanto, E. Bioaccumulation of nickel by five wild plant species on nickel-contaminated soil. IOSR J. Eng. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Effect of nickel and grafting combination on yield, fruit quality, antioxidative enzyme activities, lipid peroxidation, and mineral composition of tomato. J. Plant Nutr. Soil Sci. 2015, 178, 848–860. [Google Scholar] [CrossRef]
- Franks, S.J.; Weber, J.J.; Aitken, S.N. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 2014, 7, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Munzi, S.; Pirintsos, S.A.; Loppi, S. Chlorophyll degradation and inhibition of polyamine biosynthesis in the lichen Xanthoria parietina under nitrogen stress. Ecotoxicol. Environ. Saf. 2009, 72, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Alamri, S.; Khan, M.N.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mater. 2020, 398, 122882–122955. [Google Scholar] [CrossRef]
- Ahmed, N.; Habib, U.; Younis, U.; Irshad, I.; Danish, S.; Rahi, A.A.; Munir, T.M. Growth, chlorophyll content and productivity responses of maize to magnesium sulphate application in calcareous soil. Open Agric. 2020, 5, 792–800. [Google Scholar] [CrossRef]
- Ahmad, P.; Tripathi, D.K.; Deshmukh, R.; Pratap Singh, V.; Corpas, F.J. Revisiting the role of ROS and RNS in plants under changing environment. Environ. Exp. Bot. 2019, 161, 1–3. [Google Scholar] [CrossRef]
- Gajewska, E.; Bernat, P.; Długoński, J.; Skłodowska, M. Effect of nickel on membrane integrity, lipid peroxidation and fatty acid composition in wheat seedlings. J. Agron Crop Sci. 2012, 198, 286–294. [Google Scholar] [CrossRef]
- Mir, M.A.; Sirhindi, G.; Alyemeni, M.N.; Alam, P.; Ahmad, P. Jasmonic acid improves growth performance of soybean under nickel toxicity by regulating nickel uptake, redox balance, and oxidative stress metabolism. J. Plant Growth Regul. 2018, 37, 1195–1209. [Google Scholar] [CrossRef]
- Alsahli, A.A.; Bhat, J.A.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Hydrogen Sulfide (H2S) Mitigates Arsenic (As)-Induced Toxicity in Pea (Pisum sativum L.) Plants by Regulating Osmoregulation, Antioxidant Defense System, Ascorbate Glutathione Cycle and Glyoxalase System. J. Plant Growth Regul. 2021, 40 (Suppl. S1), 2515–2531. [Google Scholar] [CrossRef]
- Sachan, P.; Lal, N. An Overview of Nickel (Ni2+) Essentiality, Toxicity and Tolerance Strategies in Plants. Asian J. Biol. 2017, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.-Q.; Liu, W.-X.; Hao, J.-Y.; Fan, D.-N.; Chen, L.-M.; Xu, H.-N.; Li, K.-Z. Measuring the expression and activity of the CAT enzyme to determine Al resistance in soybean. Plant Physiol. Biochem. 2019, 144, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Agrawal, V. Elucidation of zinc and copper induced oxidative stress, DNA damage and activation of defence system during seed germination in Cassia angustifolia Vahl. Environ. Exp. Bot. 2016, 125, 31–41. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Tanveer, M. An Overview of Nickel Toxicity in Plants. In Metal Toxicology Handbook; CRC Press: Boca Raton, FL, USA, 2021; pp. 465–474. [Google Scholar]
- Ishtiaq, S.; Mahmood, S.; Athar, M. Alteration of macronutrients, metal translocation and bioaccumulation as potential indicators of nickel tolerance in three Vigna species. Adv. Environ. Res. 2014, 3, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Callahan, D.L.; Baker, A.J.; Kolev, S.D.; Wedd, A.G. Metal ion ligands in hyperaccumulating plants. JBIC J. Biol. Inorg. Chem. 2006, 11, 2–12. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Hazrati, N. Calcium sensing and signaling in plants during metal/metalloid stress. In Metal and Nutrient Transporters in Abiotic Stress; Elsevier: Amsterdam, The Netherlands, 2021; pp. 169–197. [Google Scholar]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Bloom, A.J. Metal regulation of metabolism. Curr. Opin. Chem. Biol. 2019, 49, 33–38. [Google Scholar] [CrossRef]
- Estravis-Barcala, M.; Mattera, M.G.; Soliani, C.; Bellora, N.; Opgenoorth, L.; Heer, K.; Arana, M.V. Molecular bases of responses to abiotic stress in tress. J. Exp. Biol. 2020, 71, 3765–3779. [Google Scholar]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Gen. Mol. Res. 2017, 40, 373–386. [Google Scholar]





| Attributes | G. asiatica L. | S. cumini (L.) Skeels | T. indica L. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni µg/kg | ||||||||||||
| Control | 10 | 20 | 40 | Control | 10 | 20 | 40 | Control | 10 | 20 | 40 | |
| Shoot fresh weight (g) | 3.14 a ± 0.10 | 2.65 a ± 0.30 | 1.86 b ± 0.06 | 0.95 c ± 0.14 | 4.03 a ± 0.89 | 1.17 c ± 0.21 | 1.78 c ± 0.43 | 0.92 c ± 0.32 | 2.81 a ± 0.05 | 2.12 b ± 0.09 | 1.61 c ± 0.07 | 1.21 d ± 0.04 |
| Shoot dry weight (g) | 1.12 a ± 0.07 | 0.98 a ± 0.07 | 0.49 b ± 0.05 | 0.31 b ± 0.02 | 1.10 ab ± 0.13 | 0.76 bc ± 0.29 | 0.94 bc ± 0.14 | 0.29 c ± 0.05 | 0.93 a ± 0.04 | 0.71 b ± 0.03 | 0.40 c ± 0.01 | 0.31 d ± 0.02 |
| Root fresh weight (g) | 2.23 a ± 0.14 | 1.64 b ± 0.12 | 1.40 b ± 0.13 | 0.68 c ± 0.10 | 2.28 a ± 0.38 | 1.74 ab ± 0.28 | 1.33 bc ± 0.39 | 0.71 c ± 0.12 | 2.13 a ± 0.10 | 2.03 a ± 0.01 | 1.69 b ± 0.06 | 1.14 c ± 0.04 |
| Root dry weight (g) | 0.29 a ± 0.04 | 0.24 ab ± 0.05 | 0.21 ab ± 0.05 | 0.14 b ± 0.01 | 0.21 b ± 0.05 | 0.28 b ± 0.02 | 0.22 b ± 0.05 | 0.13 b ± 0.04 | 0.32 a ± 0.01 | 0.24 b ± 0.02 | 0.19 c ± 0.01 | 0.12 d ± 0.01 |
| Shoot length (cm) | 12.87 a ± 1.60 | 8.97 b ± 0.98 | 7.80 b ± 0.26 | 7.33 b ± 0.74 | 26.17 a ± 2.99 | 21.23 a ± 0.91 | 21.77 a ± 0.15 | 20.80 a ± 1.05 | 27.73 a ± 3.46 | 24.00 a ± 1.48 | 23.00 a ± 0.64 | 24.53 a ± 0.82 |
| Root length (cm) | 11.43 a ± 2.05 | 7.33 a ± 0.33 | 13.73 a ± 4.09 | 11.40 a ± 2.94 | 12.43 a ± 2.49 | 10.60 a ± 3.63 | 11.77 a ± 3.72 | 13.23 a ± 2.95 | 14.70 a ± 2.59 | 8.90 a ± 2.28 | 5.77 a ± 1.19 | 11.87 a ± 2.76 |
| (a) | ||||||
| Attributes | G. asiatica L. | S. cumini (L.) Skeels | T. indica L. | |||
| Shoot fresh weight (g) | −0.48 | −0.32 | −0.44 | |||
| Shoot dry weight (g) | −0.71 * | −0.67 * | −0.70 * | |||
| Root fresh weight (g) | −0.45 | −0.38 | −0.32 | |||
| Root dry weight (g) | −0.54 * | −0.51 * | −0.61 * | |||
| Shoot length (g) | −0.33 | −0.41 | −0.39 | |||
| Root length (g) | −0.51 | −0.56 | −0.55 | |||
| Leaf number | 0.48 | 0.42 | 0.35 | |||
| Leaf area (cm2) | 0.38 | 0.40 | 0.53 | |||
| Chlorophyll a (µg g−1 FW) | −0.36 | −0.44 | −0.47 | |||
| Chlorophyll b (µg g−1 FW) | −0.22 | −0.29 | −0.33 | |||
| (b) | ||||||
| Tissues | Leaves | Roots | Leaves | Roots | Leaves | Roots |
| Na+ (mg kg−1) | −0.21 | −0.32 | −0.32 | −0.21 | −0.30 | −0.19 |
| K+ (mg kg−1) | −0.39 | −0.36 | −0.31 | −0.23 | −0.26 | −0.21 |
| Ca2+ (mg kg−1) | −0.36 | −0.33 | −0.49 | −0.56 * | −0.22 | −0.24 |
| Mg2+ (mg kg−1) | 0.62 * | 0.44 | 0.51 | 0.38 | 0.41 | 0.29. |
| MDA (µ mol mg −1 protein) | 0.31 | 0.38 | 0.26 | 0.32 | 0.24 | 0.29 |
| SOD (U mg−1 protein) | 0.25 | 0.47 | 0.21 | 0.34 | 0.21 | 0.38 |
| CAT (U mg−1 protein) | 0.37 | 0.48 | 0.23 | 0.29 | 0.57 * | 0.67 * |
| POD (U mg−1 protein) | 0.71 * | 0.67 * | 0.46 | 0.52 | 0.51 | 0.62 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahra, S.; Noreen, S.; Abid, R.; Akram, A.; Mahmood, S.; Shah, T.; Alsahli, A.A. Comparative Morphology and Biochemical Analysis of Nickel Toxicity in Minor Fruit Species (Grewia asiatica L., Syzgium cumini (L.) Skeels and Tamarindus indica L.). Agriculture 2022, 12, 323. https://doi.org/10.3390/agriculture12030323
Zahra S, Noreen S, Abid R, Akram A, Mahmood S, Shah T, Alsahli AA. Comparative Morphology and Biochemical Analysis of Nickel Toxicity in Minor Fruit Species (Grewia asiatica L., Syzgium cumini (L.) Skeels and Tamarindus indica L.). Agriculture. 2022; 12(3):323. https://doi.org/10.3390/agriculture12030323
Chicago/Turabian StyleZahra, Saman, Sibgha Noreen, Rafia Abid, Ahmed Akram, Seema Mahmood, Tariq Shah, and Abdulaziz Abdulla Alsahli. 2022. "Comparative Morphology and Biochemical Analysis of Nickel Toxicity in Minor Fruit Species (Grewia asiatica L., Syzgium cumini (L.) Skeels and Tamarindus indica L.)" Agriculture 12, no. 3: 323. https://doi.org/10.3390/agriculture12030323