Biochar Improves Sustainability of Green Roofs via Regulate of Soil Microbial Communities
Abstract
:1. Introduction
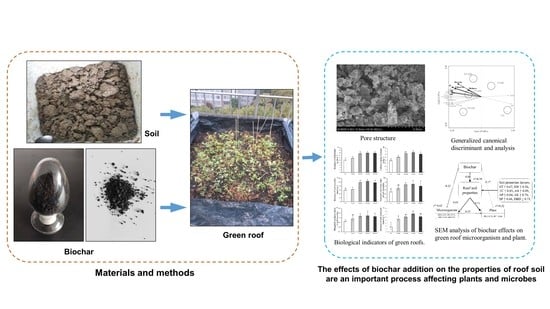
2. Materials and Methods
2.1. Study Area and Materials
2.2. Experimental Design
2.3. Sampling Procedure and Determine Property
2.4. Statistical Analysis
3. Results
3.1. Biochar Adjusted Physical and Chemical Properties of Roof Soil
3.2. Biochar Improved the Biological Characteristics of the Green Roof
4. Discussion
4.1. Influence Mechanism of Biochar on Physical Properties of the Roof Soil
4.2. Influence Mechanism of Biochar on Nutrients of the Roof Soil
4.3. Effect of Biochar on Biological Traits of the Roof Soil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, T. City of Atlanta green roof demonstration project. In Proceedings of the Chicago: First Annual Greening Rooftops for Sustainable Communities Conference, Awards and Trade Show, Chicago, IL, USA, 29–30 May 2003; pp. 29–30. [Google Scholar]
- Cao, C.T.; Farrell, C.; Kristiansen, P.E.; Rayner, J.P. Biochar makes green roof soils lighter and improves water supply to plants. Ecol. Eng. 2014, 71, 368–374. [Google Scholar] [CrossRef]
- Dvorak, B.; Volder, A. Green roof vegetation for North American ecoregions: A literature review. Landsc. Urban Plan. 2010, 96, 197–213. [Google Scholar] [CrossRef]
- Getter, K.L.; Rowe, D.B. Substrate depth influences Sedum plant community on a green roof. HortScience 2009, 44, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Oberndorfer, E.; Lundholm, J.; Bass, B.; Coffman, R.R.; Doshi, H.; Dunnett, N.; Gaffin, S.; Köhler, M.; Liu, K.; Rowe, A.B. Green roofs as urban ecosystems: Ecological structures, functions, and services. Bioscience 2007, 57, 823–833. [Google Scholar] [CrossRef]
- Williams, N.S.; Rayner, J.P.; Raynor, K.J. Green roofs for a wide brown land: Opportunities and barriers for rooftop greening in Australia. Urban For. Urban Green. 2010, 9, 245–251. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Q.; Gong, P. Quantifying air pollution removal by green roofs in Chicago. Atmos. Environ. 2008, 42, 7266–7273. [Google Scholar] [CrossRef]
- Brenneisen, S. The benefits of biodiversity from green roofs: Key design consequences. In Proceedings of the 1st North American Green Roof Conference, Chicago, IL, USA, 29–30 May 2003; pp. 323–329. Available online: https://www.researchgate.net/publication/313050350 (accessed on 15 May 2021).
- Molineux, C.J.; Gange, A.C.; Connop, S.P.; Newport, D.J. Using recycled aggregates in green roof substrates for plant diversity. Ecol. Eng. 2015, 82, 596–604. [Google Scholar] [CrossRef]
- Macivor, J.S.; Lundholm, J. Performance evaluation of native plants suited to extensive green roof conditions in a maritime climate. Ecol. Eng. 2011, 37, 407–417. [Google Scholar] [CrossRef]
- Molineux, C.J.; Fentiman, C.H.; Gange, A.C. Characterising alternative recycled waste materials for use as green roof growing media in the U.K. Ecol. Eng. 2009, 35, 1507–1513. [Google Scholar] [CrossRef]
- Molineux, C.J.; Gange, A.C.; Newport, D.J. Using soil microbial inoculations to enhance substrate performance on extensive green roofs. Sci. Total Environ. 2017, 580, 846–856. [Google Scholar] [CrossRef]
- Wolf, D.; Lundholm, J.T. Water uptake in green roof microcosms: Effects of species and water availability. Ecol. Eng. 2008, 33, 179–186. [Google Scholar] [CrossRef]
- Dunnett, N.; Kingsbury, N. Planting Green Roofs and Living Walls, 2nd ed.; Timber Press: Portland, OR, USA, 2010. [Google Scholar]
- Molineux, C.J.; Connop, S.P.; Gange, A.C. Manipulating soil microbial communities in extensive green roof substrates. Sci. Total Environ. 2014, 493, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.B.; Getter, K.L.; Durhman, A.K. Effect of green roof media depth on crassulacean plant succession over seven years. Landsc. Urban Plan. 2012, 104. [Google Scholar] [CrossRef]
- Chen, H.; Ma, J.; Wei, J.; Gong, X.; Yu, X.; Guo, H.; Zhao, Y. Biochar increases plant growth and alters microbial communities via regulating the moisture and temperature of green roof substrates. Sci. Total Environ. 2018, 635, 333–342. [Google Scholar] [CrossRef]
- Luo, H.; Liu, X.; Anderson, B.C.; Zhang, K.; Li, X.; Huang, B.; Chen, F. Carbon sequestration potential of green roofs using mixed-sewage-sludge soil in Chengdu World Modern Garden City. Ecol. Indic. 2015, 49, 247–259. [Google Scholar] [CrossRef]
- Chen, H.; Ma, J.; Wang, X.; Xu, P.; Zheng, S.; Zhao, Y. Effects of Biochar and Sludge on Carbon Storage of Urban Green Roofs. Forests 2018, 9, 413. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2013, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L. Enhanced pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, L.; Wang, Z.; Su, M.; Li, Z. Evaluating the protection of bacteria from extreme cd (ii) stress by p-enriched biochar. Environ. Pollut. 2010, 263. [Google Scholar] [CrossRef]
- Kimetu, J.M.; Lehmann, J. Stability and stabilisation of biochar and green manure in soil with different organic carbon contents. Soil Res. 2010, 48, 577–585. [Google Scholar] [CrossRef]
- Shinogi, Y.; Kanri, Y. Pyrolysis of plant, animal and human waste: Physical and chemical characterization of the pyrolytic products. Bioresour. Technol. 2003, 90, 241–247. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaseret, B.; Jindo, K. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, X.D.; Zhao, L.; Xu, X.Y.; Harris, W. Phosphorus release from dairy manure, the manure-derived biochar, and their amended soil: Effects of phosphorus nature and soil property. J. Environ. Qual. 2017, 43, 1504. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, L.; Rivier, P.A.; Mondelli, D.; Miano, T.; Joner, E.J. Assessment of addition of biochar to filtering mixtures for potential water pollutant removal. Environ. Sci. Pollut. Res. 2014, 25, 2167–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jindo, K.; Sánchez-Monedero, M.A.; Hernández, T.; García, C.; Furukawa, T.; Matsumoto, K.; Sonoki, T.; Bastida, F. Biochar influences the microbial community structure during manure composting with agricultural wastes. Sci. Total Environ. 2012, 416, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Chen, B.L.; Zhu, L.Z.; Xing, B.S. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Coomes, O.T.; Miltner, B.C. Indigenous charcoal and biochar production: Potential for soil improvement under shifting cultivation systems. Land Degrad. Dev. 2017, 28, 811–821. [Google Scholar] [CrossRef]
- FLL. Guidelines for the Planning, Execution and Upkeep of Green-Roof Sites (English Version); Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau e.V.: Troisdorf, Germany, 2002; pp. 1–97. [Google Scholar]
- AQSIQ (General Administration of Quality Supervision, Inspection and Quarantine of the China); SAC (Standardization Administration of China). The Disposal of Sludge from Municipal Wastewater Treatment Plant—The Quality of Sludge Used in Gardens or Parks (GB/T 23486-2009). 2009; pp. 1–8. Available online: http://www.jianbiaoku.com/webarbs/book/58530/1100138.shtml (accessed on 15 May 2021).
- Handreck, K.; Black, N.; Handreck, K.; Black, N. Growing Media for Ornamental Plants and Turf; UNSW Press: Randwick, Australia, 2002. [Google Scholar]
- Nelson, D.W.; Sommers, L.E.; Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. 1982, 9, 961–1010. [Google Scholar]
- Sparks, D.L.; Page, A.; Helmke, P.; Loeppert, R.; Soltanpour, P.; Tabatabai, M.; Johnston, C.; Sumner, M. Methods of Soil Analysis, Part 3, Chemical Methods; Soil Science Society of America Madison: Madison, WI, USA, 1996. [Google Scholar]
- Watanabe, F.S.; Olsen, S.R. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil1. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Knudsen, D.; Peterson, G.; Pratt, P. Lithium, sodium, and potassium. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Kenney, D.R., Eds.; American Society of Agronomy, Soil Science Society of American: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- Mitchell, C.C.; Huluka, G. Potassium Dynamics in US Coastal Plain Soils. Commun. Soil Sci. Plant Anal. 2016, 47 (Suppl. 1), 54–63. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y. Effects of nitrogen enrichment on belowground communities in grassland: Relative role of soil nitrogen availability vs. soil acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Friendly, M.; Fox, J.; Friendly, M.M. Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis. R Package Candisc Version: 0.6-5. 2013. Available online: https://cran.r-project.org/web/packages/candisc/candisc.pdf (accessed on 15 May 2021).
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Barnes, R.T.; Gallagher, M.E.; Masiello, C.A.; Liu, Z.; Dugan, B. Biochar-Induced Changes in Soil Hydraulic Conductivity and Dissolved Nutrient Fluxes Constrained by Laboratory Experiments. PLoS ONE 2014, 9, e108340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berndtsson, J.C.; Bengtsson, L.; Jinno, K. Runoff water quality from intensive and extensive vegetated roofs. Ecol. Eng. 2009, 35, 369–380. [Google Scholar] [CrossRef]
- Beck, D.A.; Johnson, G.R.; Spolek, G.A. Amending greenroof soil with biochar to affect runoff water quantity and quality. Environ. Pollut. 2011, 159, 2111. [Google Scholar] [CrossRef]
- Kuoppamäki, K.; Lehvävirta, S. Mitigating nutrient leaching from green roofs with biochar. Landsc. Urban Plan. 2016, 152, 39–48. [Google Scholar] [CrossRef]
- Curtin, D.; Trolove, S. Predicting pH buffering capacity of New Zealand soils from organic matter content and mineral characteristics. Soil Res. 2013, 51, 494. [Google Scholar] [CrossRef]
- Liu, T.; Guo, R.; Ran, W.; Whalen, J.K.; Li, H. Body size is a sensitive trait-based indicator of soil nematode community response to fertilization in rice and wheat agroecosystems. Soil Biol. Biochem. 2015, 88, 275–281. [Google Scholar] [CrossRef]
- Cross, A.; Sohi, S.P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Lehmann, J.; Kinyangi, J.; Smernik, R.; Riha, S.J.; Engelhard, M.H. Long-term black carbon dynamics in cultivated soil. Biogeochemistry 2009, 92, 163–176. [Google Scholar] [CrossRef]
- Watzinger, A.; Feichtmair, S.; Kitzler, B.; Zehetner, F.; Kloss, S.; Wimmer, B.; Zechmeister-Boltenstern, S.; Soja, G. Soil microbial communities responded to biochar application in temperate soils and slowly metabolized 13 C-labelled biochar as revealed by 13 C PLFA analyses: Results from a short-term incubation and pot experiment. Eur. J. Soil Sci. 2014, 65, 40–51. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangia, J.; Grossmana, J.; O’Neilla, B.; Skjemstadb, J.O.; Thiesa, J.; Luizãoc, F.J.; Petersend, J.; et al. Black carbon increases cation exchange capacity in soil. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Kolton, M.; Harel, Y.M.; Pasternak, Z.; Graber, E.R.; Elad, Y.; Cytryn, E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 2011, 77, 4924–4930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruun, E.W.; Petersen, C.T.; Hansen, E.; Holm, J.K.; Hauggaardnielsen, H. Biochar amendment to coarse sandy subsoil improves root growth and increases water retention. Soil Use Manag. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- Mulcahy, D.N.; Mulcahy, D.L.; Dietz, D. Biochar soil amendment increases tomato seedling resistance to drought in sandy soils. J. Arid Environ. 2013, 88, 222–225. [Google Scholar] [CrossRef]



 ); 5%SB, soil and 5% biochar (
); 5%SB, soil and 5% biochar (  ); 10%SB, soil and 10% biochar (
); 10%SB, soil and 10% biochar (  ); 15%SB, soil and 15% biochar (
); 15%SB, soil and 15% biochar (  ) and 20%SB, soil and 20% biochar (
) and 20%SB, soil and 20% biochar (  ). (A–D) Total carbon (g kg−1), total nitrogen (g kg−1), total phosphorus (g kg−1) and total potassium (g kg−1) of the roof soil, respectively.
). (A–D) Total carbon (g kg−1), total nitrogen (g kg−1), total phosphorus (g kg−1) and total potassium (g kg−1) of the roof soil, respectively.
 ); 5%SB, soil and 5% biochar (
); 5%SB, soil and 5% biochar (  ); 10%SB, soil and 10% biochar (
); 10%SB, soil and 10% biochar (  ); 15%SB, soil and 15% biochar (
); 15%SB, soil and 15% biochar (  ) and 20%SB, soil and 20% biochar (
) and 20%SB, soil and 20% biochar (  ). (A–D) Total carbon (g kg−1), total nitrogen (g kg−1), total phosphorus (g kg−1) and total potassium (g kg−1) of the roof soil, respectively.
). (A–D) Total carbon (g kg−1), total nitrogen (g kg−1), total phosphorus (g kg−1) and total potassium (g kg−1) of the roof soil, respectively.

| Substrate | BD (kg L−1) | pH | TC (g kg−1) | TOC (g kg−1) | TN (g kg−1) | TK (g kg−1) | TP (g kg−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| Soil | 1.12 ± 0.07 | 8.32 ± 0.01 | 11.36 ± 0.13 | 9.59 ± 0.05 | 1.00 ± 0.02 | 1.36 ± 0.01 | 1.21 ± 0.03 | ||
| Biochar | 0.97 ± 0.09 | 6.87 ± 0.02 | 243.38 ± 0.38 | 195.24 ±0.11 | 1.73 ± 0.03 | 0.27 ± 0.01 | 1.71 ± 0.03 | ||
| Metal contents (mg kg−1) of sludge biochar | |||||||||
| Biochar | Cr | Cd | Ni | Pb | Cu | Zn | As | Hg | B |
| 172 | 1.42 | 3.3 | 55.2 | 324 | 1420 | 1.10 | 0.296 | 56.2 | |
| Treatments | DBD (kg L−1) | WHC (%) | SP (%) | pH | TC (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) |
| CK | 1.02 ± 0.02 a | 43.56 ± 0.17 e | 58.32 ± 0.06 d | 8.17 ± 0.03 a | 19.63 ± 0.15 e | 1.38 ± 0.03 e | 0.96 ± 0.06 c | 1.15 ± 0.06 d |
| 5%SB | 0.98 ± 0.03 b | 49.66 ± 0.12 d | 61.38 ± 0.05 c | 7.67 ± 0.02 b | 24.57 ± 0.08 d | 1.76 ± 0.01 d | 1.02 ± 0.03 c | 1.23 ± 0.03 cd |
| 10%SB | 0.95 ± 0.02 bc | 55.72 ± 0.21 c | 62.77 ± 0.08 b | 7.32 ± 0.01 c | 29.65 ± 0.16 c | 2.53 ± 0.02 c | 1.15 ± 0.02 b | 1.32 ± 0.06 bc |
| 15%SB | 0.93 ± 0.01 cd | 57.23 ± 0.18 b | 63.63 ± 0.12 a | 7.28 ± 0.02 cd | 28.99 ± 0.12 b | 2.75 ± 0.05 b | 1.21 ± 0.03 ab | 1.39 ± 0.07 b |
| 20%SB | 0.91 ± 0.02 d | 59.76 ± 0.22 a | 63.76 ± 0.13 a | 7.25 ± 0.03 d | 31.16 ± 0.11 a | 2.97 ± 0.06 a | 1.23 ± 0.01 a | 1.56 ± 0.03 a |
| Treatments | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | CEC (coml kg−1) | Soil Water (%) | Soil Temperature (°C) | SWM (%) | SMT (°C) |
| CK | 9.32 ± 0.15 e | 6.43 ± 0.06 e | 10.12 ± 0.16 e | 9.17 ± 0.66 d | 31.63 ± 0.32 d | 11.62 ± 0.12 e | 46.6 ± 0.26 a | 21.5 ± 0.25 e |
| 5%SB | 12.11 ± 0.08 d | 6.83 ± 0.03 d | 10.82 ± 0.11 d | 12.66 ± 1.06 c | 33.71 ± 0.21 c | 12.15 ± 0.15 d | 45.3 ± 0.17 c | 23.8 ± 0.19 d |
| 10%SB | 13.23 ± 0.16 c | 7.71 ± 0.08 c | 11.62 ± 0.09 c | 14.26 ± 1.02 b | 37.96 ± 0.63 b | 13.72 ± 0.29 c | 41.3 ± 0.11 e | 30.7 ± 0.16 a |
| 15%SB | 15.65 ± 0.06 b | 8.11 ± 0.11 b | 12.23 ± 0.12 b | 15.57 ± 0.95 ab | 38.65 ± 0.56 b | 15.23 ± 0.18 b | 43.8 ± 0.16 d | 29.6 ± 0.22 b |
| 20%SB | 17.72 ± 0.06 a | 8.26 ± 0.09 a | 13.73 ± 0.22 a | 16.13 ± 0.58 a | 49.52 ± 0.37 a | 16.86 ± 0.26 a | 44.9 ± 0.23 b | 27.7 ± 0.32 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Du, X.; Lai, M.; Nazhafati, M.; Li, C.; Qi, W. Biochar Improves Sustainability of Green Roofs via Regulate of Soil Microbial Communities. Agriculture 2021, 11, 620. https://doi.org/10.3390/agriculture11070620
Chen H, Du X, Lai M, Nazhafati M, Li C, Qi W. Biochar Improves Sustainability of Green Roofs via Regulate of Soil Microbial Communities. Agriculture. 2021; 11(7):620. https://doi.org/10.3390/agriculture11070620
Chicago/Turabian StyleChen, Haoming, Xianfeng Du, Mengqi Lai, Muhanmaitijiang Nazhafati, Chen Li, and Weicong Qi. 2021. "Biochar Improves Sustainability of Green Roofs via Regulate of Soil Microbial Communities" Agriculture 11, no. 7: 620. https://doi.org/10.3390/agriculture11070620