Changes in Respiratory Muscle Strength Following Cardiac Rehabilitation for Prognosis in Patients with Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Patient Characteristics
2.3. Pulmonary and Respiratory Muscle Functions
2.4. End-Points
2.5. Cardiac Rehabilitation Program
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
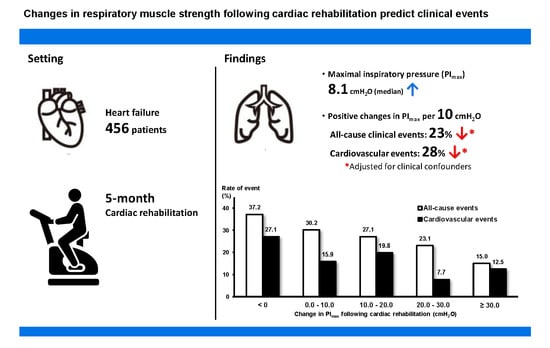
3.2. Relationships between Change in Respiratory Muscle Strength and Adverse Clinical Events
3.3. Poisson Regression Models for Clinical Events
3.4. Unadjusted Rates of Clinical Events
3.5. Predictive Significance of Changes in Clinical Variables Following Cardiac Rehabilitation for Clinical Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hunt, S.A.; Baker, D.W.; Chin, M.H.; Cinquegrani, M.P.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Goldstein, S.; Gregoratos, G.; Jessup, M.L.; et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation 2001, 104, 2996–3007. [Google Scholar] [PubMed] [Green Version]
- Kelley, R.C.; Ferreira, L.F. Diaphragm abnormalities in heart failure and aging: Mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail. Rev. 2017, 22, 191–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stassijns, G.; Gayan-Ramirez, G.; De Leyn, P.; Verhoeven, G.; Herijgers, P.; de Bock, V.; Dom, R.; Lysens, R.; Decramer, M. Systolic ventricular dysfunction causes selective diaphragm atrophy in rats. Am. J. Respir. Crit. Care Med. 1998, 158, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Laghi, F.; Tobin, M.J. Disorders of the respiratory muscles. Am. J. Respir. Crit. Care Med. 2003, 168, 10–48. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.S.; Rolim, N.P.; Fischer, T.; Baekkerud, F.H.; Medeiros, A.; Werner, S.; Bronstad, E.; Rognmo, O.; Mangner, N.; Linke, A.; et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur. J. Heart Fail. 2015, 17, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, N.; Masuda, T.; Kamiya, K.; Matsuzawa, R.; Nozaki, K.; Maekawa, E.; Noda, C.; Yamaoka-Tojo, M.; Ako, J. Respiratory muscle weakness increases dead-space ventilation ratio aggravating ventilation-perfusion mismatch during exercise in patients with chronic heart failure. Respirology 2019, 24, 154–161. [Google Scholar] [CrossRef]
- Hamazaki, N.; Kamiya, K.; Matsuzawa, R.; Nozaki, K.; Ichikawa, T.; Tanaka, S.; Nakamura, T.; Yamashita, M.; Maekawa, E.; Noda, C.; et al. Prevalence and prognosis of respiratory muscle weakness in heart failure patients with preserved ejection fraction. Respir. Med. 2019, 161, 105834. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Smart, N.A.; Giallauria, F.; Dieberg, G. Efficacy of inspiratory muscle training in chronic heart failure patients: A systematic review and meta-analysis. Int. J. Cardiol. 2013, 167, 1502–1507. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Habedank, D.; Meyer, F.J.; Hetzer, R.; Anker, S.D.; Ewert, R. Relation of respiratory muscle strength, cachexia and survival in severe chronic heart failure. J. Cachexia Sarcopenia Muscle 2013, 4, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Izawa, H.; Yoshida, T.; Ikegame, T.; Izawa, K.P.; Ito, Y.; Okamura, H.; Osada, N.; Kinugawa, S.; Kubozono, T.; Kono, Y.; et al. Standard Cardiac Rehabilitation Program for Heart Failure. Circ. J. 2019, 83, 2394–2398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.J.; Sung, S.H.; Cheng, H.M.; Huang, W.M.; Wu, C.L.; Huang, C.J.; Hsu, P.F.; Yeh, J.S.; Guo, C.Y.; Yu, W.C.; et al. Performance of AHEAD Score in an Asian Cohort of Acute Heart Failure With Either Preserved or Reduced Left Ventricular Systolic Function. J. Am. Heart Assoc. 2017, 6, e004297. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Tojo, N.; Suga, H.; Kambe, M. Lung function testing—The Official Guideline of the Japanese Respiratory Society. Rinsho Byori 2005, 53, 77–81. [Google Scholar]
- American Thoracic Society; European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef]
- Suzuki, M.; Teramoto, S.; Sudo, E.; Ogawa, K.; Namekawa, T.; Motrita, K.; Matsuse, T.; Takizawa, H.; Ouchi, Y.; Fukuchi, Y. Age-related changes in static maximal inspiratory and expiratory pressures. Nihon Kyobu Shikkan Gakkai Zasshi 1997, 35, 1305–1311. [Google Scholar]
- Hughes, P.D.; Polkey, M.I.; Harris, M.L.; Coats, A.J.S.; Moxham, J.; Green, M. Diaphragm strength in chronic heart failure. Am. J. Respir. Crit. Care Med. 1999, 160, 529–534. [Google Scholar] [CrossRef]
- Group JCSJW. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ. J. 2014, 78, 2022–2093. [Google Scholar] [CrossRef] [Green Version]
- Piepoli, M.F.; Conraads, V.; Corra, U.; Dickstein, K.; Francis, D.P.; Jaarsma, T.; McMurray, J.; Pieske, B.; Piotrowicz, E.; Schmid, J.P.; et al. Exercise training in heart failure: From theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur. J. Heart Fail. 2011, 13, 347–357. [Google Scholar] [CrossRef]
- Meyer, F.J.; Borst, M.M.; Zugck, C.; Kirschke, A.; Schellberg, D.; Kubler, W.; Haass, M. Respiratory muscle dysfunction in congestive heart failure: Clinical correlation and prognostic significance. Circulation 2001, 103, 2153–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iversen, K.K.; Kjaergaard, J.; Akkan, D.; Kober, L.; Torp-Pedersen, C.; Hassager, C.; Vestbo, J.; Kjoller, E.; Group, E.L.F.S. The prognostic importance of lung function in patients admitted with heart failure. Eur. J. Heart Fail. 2010, 12, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.L.; Lin, I.F.; Hsieh, M.J. More Impaired Dynamic Ventilatory Muscle Oxygenation in Congestive Heart Failure than in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2019, 8, 1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, A.M.; Toledo-Arruda, A.C.; Lima, J.S.; Duarte, C.S.; Villacorta, H.; Nobrega, A.C.L. Inspiratory Muscle Training Improves Intercostal and Forearm Muscle Oxygenation in Patients With Chronic Heart Failure: Evidence of the Origin of the Respiratory Metaboreflex. J. Card. Fail. 2017, 23, 672–679. [Google Scholar] [CrossRef]
- Chiappa, G.R.; Roseguini, B.T.; Vieira, P.J.; Alves, C.N.; Tavares, A.; Winkelmann, E.R.; Ferlin, E.L.; Stein, R.; Ribeiro, J.P. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J. Am. Coll. Cardiol. 2008, 51, 1663–1671. [Google Scholar] [CrossRef] [Green Version]
- Del Buono, M.G.; Arena, R.; Borlaug, B.A.; Carbone, S.; Canada, J.M.; Kirkman, D.L.; Garten, R.; Rodriguez-Miguelez, P.; Guazzi, M.; Lavie, C.J.; et al. Exercise Intolerance in Patients With Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2209–2225. [Google Scholar] [CrossRef]
- Jaenisch, R.B.; Hentschke, V.S.; Quagliotto, E.; Cavinato, P.R.; Schmeing, L.A.; Xavier, L.L.; Dal Lago, P. Respiratory muscle training improves hemodynamics, autonomic function, baroreceptor sensitivity, and respiratory mechanics in rats with heart failure. J. Appl. Physiol. (1985) 2011, 111, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Tager, T.; Hanholz, W.; Cebola, R.; Frohlich, H.; Franke, J.; Doesch, A.; Katus, H.A.; Wians, F.H., Jr.; Frankenstein, L. Minimal importance for 6-minute walk test distances among patients with chronic heart failure. Int. J. Cardiol. 2014, 176, 94–98. [Google Scholar] [CrossRef]
- Clark, A.L.; Kalra, P.R.; Petrie, M.C.; Mark, P.B.; Tomlinson, L.A.; Tomson, C.R.V. Change in renal function associated with drug treatment in heart failure: National guidance. Heart 2019, 105, 904–910. [Google Scholar] [CrossRef]
- Dall’Ago, P.; Chiappa, G.R.; Guths, H.; Stein, R.; Ribeiro, J.P. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: A randomized trial. J. Am. Coll. Cardiol. 2006, 47, 757–763. [Google Scholar] [CrossRef] [Green Version]





| Groups | Overall | ⊿PImax ≤ 0 cmH2O | ⊿PImax > 0 cmH2O | p Value |
|---|---|---|---|---|
| n | 456 | 130 | 326 | |
| Age, y.o. | 68 (57–75) | 69 (59–74) | 67 (55–75) | 0.285 |
| Gender, n (%) | ||||
| Female | 144 (31.6) | 38 (29.2) | 106 (32.5) | 0.577 |
| Male | 312 (68.4) | 92 (70.8) | 220 (67.5) | |
| BMI, kg/m2 | 23.0 ± 4.1 | 22.8 ± 3.7 | 23.1 ± 4.3 | 0.478 |
| HR, beats/min | 82 ± 23 | 84 ± 25 | 81 ± 22 | 0.224 |
| sBP, mm Hg | 123 ± 29 | 123 ± 32 | 123 ± 28 | 0.839 |
| dBP, mm Hg | 72 ± 19 | 72 ± 21 | 71 ± 19 | 0.638 |
| Medical History, n (%) | ||||
| Ischemic Heart Disease | 240 (52.6) | 72 (55.4) | 168 (51.5) | 0.469 |
| Cardiomyopathy | 95 (20.8) | 25 (19.2) | 70 (21.5) | 0.702 |
| Atrial Fibrillation | 79 (17.3) | 29 (22.3) | 50 (15.3) | 0.099 |
| Hypertension | 298 (65.4) | 89 (68.5) | 209 (64.1) | 0.446 |
| Dyslipidemia | 292 (64.0) | 88 (67.7) | 204 (62.6) | 0.332 |
| Diabetes Mellitus | 174 (38.2) | 49 (37.7) | 125 (38.3) | 0.915 |
| Chronic Kidney Disease | 261 (57.4) | 79 (60.8) | 182 (56.0) | 0.401 |
| Prior Admission for HF, n (%) | 101 (22.1) | 34 (26.2) | 67 (20.6) | 0.212 |
| NYHA Class, n (%) | ||||
| II | 353 (77.8) | 103 (80.5) | 250 (76.7) | 0.452 |
| III | 101 (22.2) | 25 (19.5) | 76 (23.3) | |
| LVEF, % | 46.3 ± 15.2 | 46.4 ± 14.7 | 46.3 ± 15.4 | 0.967 |
| LVEF groups, n (%) | ||||
| <40% | 148 (33.2) | 45 (35.2) | 103 (32.4) | 0.806 |
| 40–50% | 189 (42.4) | 54 (42.2) | 135 (42.5) | |
| >50% | 109 (24.4) | 29 (22.7) | 80 (25.2) | |
| Smoking History, n (%) | 259 (56.8) | 77 (59.2) | 182 (55.8) | 0.531 |
| Pack-Years | 9.0 (0.0–35.0) | 10.0 (0.0–40.0) | 8.3 (0.0–33.0) | 0.401 |
| Medications, n (%) | ||||
| ACE-I or ARB | 392 (86.0) | 116 (89.2) | 276 (84.7) | 0.234 |
| Beta-Blockers | 374 (82.0) | 108 (83.1) | 266 (81.6) | 0.788 |
| Diuretic | 308 (67.5) | 92 (70.8) | 216 (66.3) | 0.377 |
| Hemoglobin, g/dL | 12.8 ± 2.3 | 13.0 ± 2.5 | 12.7 ± 2.2 | 0.168 |
| Albumin, g/dL | 3.7 ± 0.5 | 3.7 ± 0.5 | 3.7 ± 0.5 | 0.837 |
| Creatinine, g/dL | 0.98 (0.80–1.21) | 0.99 (0.84–1.23) | 0.96 (0.80–1.21) | 0.395 |
| eGFR, mL/min/1.73m2 | 55.7 ± 21.5 | 55.1 ± 22.2 | 56.0 ± 21.2 | 0.685 |
| BNP, pg/mL | 227.7 (113.0–435.4) | 269.7 (129.7–528.5) | 213.2 (106.7–385.8) | 0.030 |
| AHEAD Score | 1.6 ± 1.2 | 1.6 ± 1.2 | 1.6 ± 1.2 | 0.659 |
| Six-min Walk Distance, m | 446 (360–510) | 435 (355–525) | 448 (363–507) | 0.852 |
| %FVC, % | 80.0 ± 18.1 | 80.6 ± 19.4 | 79.8 ± 17.5 | 0.661 |
| FEV1/FVC, % | 77.5 ± 9.5 | 76.8 ± 8.0 | 77.8 ± 10.0 | 0.349 |
| PImax, cmH2O | 58.5 ± 27.1 | 67.5 ± 27.0 | 54.9 ± 26.4 | <0.001 |
| Respiratory Muscle Weakness, n (%) | 148 (33.0) | 26 (20.3) | 122 (38.1) | <0.001 |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| IRR | 95% CI | p Value | IRR | 95% CI | p Value | ||
| All-Cause Events | |||||||
| ⊿PImax increase of 10 cmH2O | 0.75 | 0.69–0.82 | <0.001 | 0.77 | 0.70–0.86 | <0.001 | |
| ⊿PImax | ≤0 cmH2O | 1.00 | Reference | 1.00 | Reference | ||
| >0 cmH2O | 0.52 | 0.40–0.68 | <0.001 | 0.70 | 0.52–0.93 | 0.014 | |
| Cardiovascular Events | |||||||
| ⊿PImax increase of 10 cmH2O | 0.71 | 0.63–0.79 | <0.001 | 0.72 | 0.63–0.82 | <0.001 | |
| ⊿PImax | ≤0 cmH2O | 1.00 | Reference | 1.00 | Reference | ||
| >0 cmH2O | 0.39 | 0.28–0.55 | <0.001 | 0.52 | 0.36–0.75 | <0.001 | |
| Adjusted IRR | Unit Changes | 95% CI | p Value | ||
|---|---|---|---|---|---|
| ⊿BMI | 1.03 | 1 kg/m2 | 0.95–1.11 | 0.514 | |
| ⊿HR | 1.00 | 5 beats/min | 0.99–1.01 | 0.826 | |
| ⊿sBP | 1.00 | 5 mm Hg | 0.99–1.04 | 0.856 | |
| ⊿dBP | 1.04 | 5 mm Hg | 0.99–1.10 | 0.125 | |
| ⊿Hemoglobin | 0.96 | 1 g/dL | 0.89–1.05 | 0.379 | |
| ⊿Albumin | 0.98 | 0.1 g/dL | 0.95–1.01 | 0.265 | |
| ⊿Creatinine | 0.90 | 0.1 g/dL | 0.89–0.91 | <0.001 | |
| ⊿eGFR | 1.00 | 1 mL/min/1.73m2 | 0.99–1.01 | 0.825 | |
| ⊿BNP | 1.00 | 10 pg/mL | 1.00–1.01 | 0.096 | |
| ⊿6MWD | 0.93 | 10 m | 0.91–0.95 | 0.019 | |
| ⊿%FVC | 0.98 | 5% | 0.92–1.05 | 0.595 | |
| ⊿FEV1/FVC | 1.04 | 5% | 0.95–1.14 | 0.344 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamazaki, N.; Kamiya, K.; Yamamoto, S.; Nozaki, K.; Ichikawa, T.; Matsuzawa, R.; Tanaka, S.; Nakamura, T.; Yamashita, M.; Maekawa, E.; et al. Changes in Respiratory Muscle Strength Following Cardiac Rehabilitation for Prognosis in Patients with Heart Failure. J. Clin. Med. 2020, 9, 952. https://doi.org/10.3390/jcm9040952
Hamazaki N, Kamiya K, Yamamoto S, Nozaki K, Ichikawa T, Matsuzawa R, Tanaka S, Nakamura T, Yamashita M, Maekawa E, et al. Changes in Respiratory Muscle Strength Following Cardiac Rehabilitation for Prognosis in Patients with Heart Failure. Journal of Clinical Medicine. 2020; 9(4):952. https://doi.org/10.3390/jcm9040952
Chicago/Turabian StyleHamazaki, Nobuaki, Kentaro Kamiya, Shohei Yamamoto, Kohei Nozaki, Takafumi Ichikawa, Ryota Matsuzawa, Shinya Tanaka, Takeshi Nakamura, Masashi Yamashita, Emi Maekawa, and et al. 2020. "Changes in Respiratory Muscle Strength Following Cardiac Rehabilitation for Prognosis in Patients with Heart Failure" Journal of Clinical Medicine 9, no. 4: 952. https://doi.org/10.3390/jcm9040952