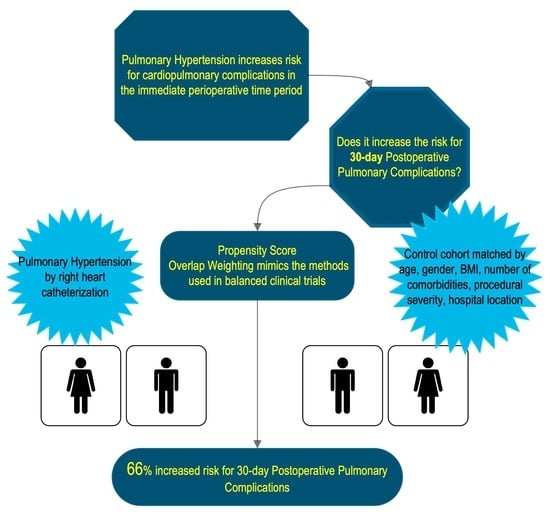
Pulmonary Hypertension and the Risk of 30-Day Postoperative Pulmonary Complications after Gastrointestinal Surgical or Endoscopic Procedures: A Retrospective Propensity Score-Weighted Cohort Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics of PH and Control Cohorts
3.2. Overlap-Weighted Outcome Analysis
3.3. Sub-Cohort Analysis: Comparisons of Pre-Capillary vs. Post-Capillary PH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| AHRQ-PPC | ICD-10 | Sub-Composite Inclusion |
|---|---|---|
| Transfusion-related acute lung injury | J95.84 | AHRQ-RF |
| Respiratory complications not elsewhere classified | J95.85 | AHRQ-RF |
| Ventilator associated pneumonia | J95.86 | AHRQ-PNA |
| Post-procedural aspiration pneumonia | J95.87 | AHRQ-ASP |
| Other complication of ventilator | J95.88 | AHRQ-RF |
| Other intraoperative complications of respiratory system, not elsewhere classified | J95.89 | AHRQ-RF |
| Other postprocedural complications and disorders of respiratory system, not elsewhere classified | J95.90 | AHRQ-RF |
| Acute respiratory failure, unspecified whether with hypoxia or hypercapnia | J95.91 | AHRQ-RF |
| Respiratory failure, unspecified, unspecified whether with hypoxia or hypercapnia | J95.92 | AHRQ-RF |
| Respiratory failure, unspecified with hypoxia | J95.93 | AHRQ-RF |
| Respiratory failure, unspecified with hypercapnia | J95.94 | AHRQ-RF |
| Acute and chronic respiratory failure, unspecified whether with hypoxia or hypercapnia | J95.95 | AHRQ-RF |
| Acute and chronic respiratory failure with hypoxia | J95.96 | AHRQ-RF |
| Acute and chronic respiratory failure with hypercapnia | J95.97 | AHRQ-RF |
| Respiratory disorders in diseases classified elsewhere | J95.98 | AHRQ-RF |
| Pulmonary insufficiency following trauma and surgery | J95.99 | AHRQ-RF |
| Acute postprocedural respiratory failure | J95.100 | AHRQ-RF |
| Acute respiratory failure with hypoxia | J95.101 | AHRQ-RF |
| Acute respiratory failure with hypercapnia | J95.102 | AHRQ-RF |
| Acute pulmonary insufficiency following thoracic surgery | J95.103 | AHRQ-RF |
| Acute pulmonary insufficiency following nonthoracic surgery | J95.104 | AHRQ-RF |
| Chronic pulmonary insufficiency following surgery | J95.105 | AHRQ-RF |
| Acute and chronic postprocedural respiratory failure | J95.106 | AHRQ-RF |
| Acute respiratory distress syndrome | J95.107 | AHRQ-RF |
| Chronic respiratory failure, unspecified whether with hypoxia or hypercapnia | J95.108 | AHRQ-RF |
| Chronic respiratory failure with hypoxia | J95.109 | AHRQ-RF |
| Chronic respiratory failure with hypercapnia | J95.110 | AHRQ-RF |
| Respiratory arrest | J95.111 | AHRQ-RF |
| Pneumonia due to streptococcus pneumonia | J95.112 | AHRQ-PNA |
| Other bacterial pneumonia | J95.113 | AHRQ-PNA |
| Pneumonia due to Pseudomonas | J95.114 | AHRQ-PNA |
| Pneumonia due to streptococcus | J95.115 | AHRQ-PNA |
| Pneumonia due to staphylococcus | J95.116 | AHRQ-PNA |
| Pneumonia due to Methicillin susceptible Staphylococcus | J95.117 | AHRQ-PNA |
| Pneumonia due to Methicillin susceptible Staphylococcus aureus | J95.118 | AHRQ-PNA |
| Pneumonia due to Escherichia coli | J95.119 | AHRQ-PNA |
| Pneumonia due to other Gram-negative bacteria | J95.120 | AHRQ-PNA |
| Pneumonia due to other specified bacteria | J95.121 | AHRQ-PNA |
| Unspecified bacterial pneumonia | J95.122 | AHRQ-PNA |
| Pneumonia due to other specified infectious organisms | J95.123 | AHRQ-PNA |
| Invasive pulmonary aspergillus | J95.124 | AHRQ-PNA |
| Bronchopneumonia, unspecified organism | J95.125 | AHRQ-PNA |
| Pneumonia, unspecified organism | J95.126 | AHRQ-PNA |
| Pneumonia due to Klebsiella pneumonia | J95.127 | AHRQ-PNA |
| Pneumonia due to other streptococci | J95.128 | AHRQ-PNA |
| Pneumonitis due to solids and liquids | J95.129 | AHRQ-ASP |
| Hypostatic pneumonia, unspecified organism | J95.130 | AHRQ-PNA |
| Other ill-defined and unknown causes of morbidity and mortality | J95.131 | |
| Respiratory conditions due to chemical fumes and vapors | J95.132 | |
| Pneumonitis due to solids and liquids | J95.133 | AHRQ-ASP |
| Pneumonitis due to inhalation of food and vomit | J95.134 | AHRQ-ASP |
| Postprocedural pneumothorax | J95.135 | |
| Septic pulmonary embolism without acute cor pulmonale | J95.136 | AHRQ-PE |
| Other pulmonary embolism without acute cor pulmonale | J95.137 | AHRQ-PE |
| Air embolism following infusion, transfusion and therapeutic injection, initial encounter | J95.138 | AHRQ-PE |
| Complication of other artery following a procedure, not elsewhere classified, initial encounter | J95.139 | |
| Complication of vein following a procedure, not elsewhere classified, initial encounter | J95.140 | |
| Embolism due to cardiac prosthetic devices, implants and grafts, initial encounter | J95.141 | AHRQ-PE |
| Embolism due to cardiac prosthetic devices, implants and grafts, initial encounter | J95.142 | AHRQ-PE |
| Embolism due to vascular prosthetic devices, implants and grafts, initial encounter | J95.143 | AHRQ-PE |
| Embolism due to vascular prosthetic devices, implants and grafts, initial encounter | J95.144 | AHRQ-PE |
References
- Maron, B.A. Revised Definition of Pulmonary Hypertension and Approach to Management: A Clinical Primer. J. Am. Heart Assoc. 2023, 12, e029024. [Google Scholar] [CrossRef]
- Gelzinis, T.A. Pulmonary Hypertension in 2021: Part I-Definition, Classification, Pathophysiology, and Presentation. J. Cardio-thorac. Vasc. Anesth. 2022, 36, 1552–1564. [Google Scholar] [CrossRef]
- Ramakrishna, G.; Sprung, J.; Ravi, B.S.; Chandrasekaran, K.; McGoon, M.D. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: Predictors of perioperative morbidity and mortality. J. Am. Coll. Cardiol. 2005, 45, 1691–1699. [Google Scholar] [CrossRef]
- Price, L.C.; Martinez, G.; Brame, A.; Pickworth, T.; Samaranayake, C.; Alexander, D.; Garfield, B.; Aw, T.-C.; McCabe, C.; Mukherjee, B.; et al. Perioperative management of patients with pulmonary hypertension undergoing non-cardiothoracic, non-obstetric surgery: A systematic review and expert consensus statement. Br. J. Anaesth. 2021, 126, 774–790. [Google Scholar] [CrossRef]
- Kruthiventi, S.C.; Kane, G.C.; Sprung, J.; Weingarten, T.N.; Warner, M.E. Postoperative pulmonary complications in contemporary cohort of patients with pulmonary hypertension. Bosn. J. Basic. Med. Sci. 2019, 19, 392–399. [Google Scholar] [CrossRef]
- Duchnowski, P.; Hryniewiecki, T.; Kuśmierczyk, M.; Szymański, P. Right ventricular systolic pressure as a predictive factor for postoperative pneumonia in patients with valvular heart disease. Kardiol. Pol. 2019, 77, 969–971. [Google Scholar] [CrossRef]
- Aasen, D.M.; Bronsert, M.R.; Rozeboom, P.D.; Colborn, K.L.; Henderson, W.G.; Lambert-Kerzner, A.; Hammermeister, K.E.; Meguid, R.A. Relationships between predischarge and postdischarge infectious complications, length of stay, and unplanned readmissions in the ACS NSQIP database. Surgery 2020, 169, 325–332. [Google Scholar] [CrossRef]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef]
- Protopapa, K.L.; Simpson, J.C.; E Smith, N.C.; Moonesinghe, S.R. Development and validation of the Surgical Outcome Risk Tool (SORT). Br. J. Surg. 2014, 101, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, J.B.; Anderson, G.F.; Gerstenblith, G.; Weller, W.; Niefeld, M.; Herbert, R.; Wu, A.W. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J. Am. Coll. Cardiol. 2003, 42, 1226–1233. [Google Scholar] [CrossRef]
- Murad, K.; Kitzman, D.W. Frailty and multiple comorbidities in the elderly patient with heart failure: Implications for management. Heart Fail. Rev. 2012, 17, 581–588. [Google Scholar] [CrossRef]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Thomas, L.; Li, F.; Pencina, M. Using Propensity Score Methods to Create Target Populations in Observational Clinical Research. JAMA 2020, 323, 466–467. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.-C.; Gibbs, J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- Hill, N.S.; Roberts, K.R.; Preston, I.R. Postoperative Pulmonary Hypertension: Etiology and Treatment of a Dangerous Complication. Respir. Care 2009, 54, 958–968. [Google Scholar] [CrossRef]
- Margraf, A.; Ludwig, N.; Zarbock, A.; Rossaint, J. Systemic Inflammatory Response Syndrome After Surgery: Mechanisms and Protection. Anesth. Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef]
- Koirala, U.; Thapa, P.B.; Joshi, M.R.; Singh, D.R.; Sharma, S.K. Systemic Inflammatory Response Syndrome following Gastrointestinal Surgery. J. Nepal. Med. Assoc. 2017, 56, 221–225. [Google Scholar] [CrossRef]
- Quarck, R.; Nawrot, T.; Meyns, B.; Delcroix, M. C-reactive protein: A new predictor of adverse outcome in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 53, 1211–1218. [Google Scholar] [CrossRef]
- Baptista de Barros Ribeiro Dourado, L.P.; Santos, M.; Moreira-Gonçalves, D. Nets, pulmonary arterial hypertension, and thrombo-inflammation. J. Mol. Med. 2022, 100, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Asllanaj, B.; Benge, E.; Bae, J.; McWhorter, Y. Fluid management in septic patients with pulmonary hypertension, review of the literature. Front. Cardiovasc. Med. 2023, 10, 1096871. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Burks, M.; Kingman, M.; Stewart, T. Volume Management in Pulmonary Arterial Hypertension Patients: An Expert Pulmonary Hypertension Clinician Perspective. Pulm. Ther. 2018, 4, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Liao, W.; Qiu, J.; Su, Q.; Fang, Y.; Gu, B. N-terminal prohormone B-type natriuretic peptide and weaning outcome in post-operative patients with pulmonary complications. J. Int. Med. Res. 2013, 41, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Pausch, C.; Coghlan, J.G.; Huscher, D.; Pittrow, D.; Grünig, E.; Staehler, G.; Vizza, C.D.; Gall, H.; Distler, O.; et al. Risk stratification and response to therapy in patients with pulmonary arterial hypertension and comorbidities: A COMPERA analysis. J. Hear. Lung Transplant. 2022, 42, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Reesink, H.J.; Tulevski, I.I.; Marcus, J.T.; Boomsma, F.; Kloek, J.J.; Noordegraaf, A.V.; Bresser, P. Brain natriuretic peptide as noninvasive marker of the severity of right ventricular dysfunction in chronic thromboembolic pulmonary hypertension. Ann. Thorac. Surg. 2007, 84, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Yap, L.B.; Ashrafian, H.; Mukerjee, D.; Coghlan, J.G.; Timms, P.M. The natriuretic peptides and their role in disorders of right heart dysfunction and pulmonary hypertension. Clin. Biochem. 2004, 37, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Messmer, A.S.; Zingg, C.; Müller, M.; Gerber, J.L.; Schefold, J.C.; Pfortmueller, C.A. Fluid Overload and Mortality in Adult Critical Care Patients—A Systematic Review and Meta-Analysis of Observational Studies*. Crit. Care Med. 2020, 48, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Price, L.C.; Montani, D.; Jais, X.; Dick, J.R.; Simonneau, G.; Sitbon, O.; Mercier, F.J.; Humbert, M. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur. Respir. J. 2010, 35, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Minai, O.A.; Venkateshiah, S.B.; Arroliga, A.C. Surgical intervention in patients with moderate to severe pulmonary arterial hy-pertension. Conn. Med. 2006, 70, 239–243. [Google Scholar]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J.; et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef]
- Lawrence, V.A.; Dhanda, R.; Hilsenbeck, S.G.; Page, C.P. Risk of pulmonary complications after elective abdominal surgery. Chest 1996, 110, 744–750. [Google Scholar] [CrossRef]
- Maron, B.A.; Brittain, E.L.; Hess, E.; Waldo, S.W.; Barón, A.E.; Huang, S.; Goldstein, R.H.; Assad, T.; Wertheim, B.M.; Alba, G.A.; et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hyper-tension: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 873–884. [Google Scholar] [CrossRef]
- Chung, L.; Liu, J.; Parsons, L.; Hassoun, P.M.; McGoon, M.; Badesch, D.B.; Miller, D.P.; Nicolls, M.R.; Zamanian, R.T. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: Identifying systemic sclerosis as a unique phenotype. Chest 2010, 138, 1383–1394. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]


| Control (N = 2005) | PH Cohort (N = 167) | p-Value * | Overall (N = 2172) | |
| Gender | ||||
| Female | 1327 (66.2%) | 104 (62.3%) | Chi-sq p = 0.348 | 1431 (65.9%) |
| Male | 678 (33.8%) | 63 (37.7%) | 741 (34.1%) | |
| Age in years | ||||
| Mean (SD) | 57.4 (17.2) | 62.2 (13.9) | t-test p < 0.001 | 57.8 (17.0) |
| Body Mass Index | ||||
| Mean (SD) | 34.3 (112) | 29.6 (8.87) | t-test p = 0.074 | 33.9 (108) |
| Missing | 24 (1.2%) | 3 (1.8%) | 27 (1.2%) | |
| Number of Elixhauser Comorbidities | ||||
| Mean (SD) | 3.22 (2.56) | 6.92 (1.98) | t-test p < 0.001 | 3.51 (2.70) |
| Ethnicity | ||||
| White | 1467 (73.2%) | 93 (55.7%) | 1560 (71.8%) | |
| Hispanic | 166 (8.3%) | 18 (10.8%) | Fisher p < 0.001 | 184 (8.5%) |
| Black | 322 (16.1%) | 47 (28.1%) | 369 (17.0%) | |
| Other | 50 (2.5%) | 9 (5.4%) | 59 (2.7%) | |
| ASA Class | ||||
| 1–2 | 612 (37.5%) | 3 (1.9%) | Chi-sq p < 0.001 | 615 (34.3%) |
| 3–4 | 1019 (62.5%) | 157 (98.1%) | 1176 (65.7%) | |
| Missing | 374 (18.7%) | 7 (4.2%) | 381 (17.5%) | |
| Procedural Severity ** | ||||
| Minor | 1 (0.0%) | 1 (0.6%) | 2 (0.1%) | |
| Intermediate | 652 (32.5%) | 113 (67.7%) | 765 (35.2%) | |
| Major | 867 (43.2%) | 26 (15.6%) | Fisher p < 0.001 | 893 (41.1%) |
| Xmajor/Complex | 485 (24.2%) | 27 (16.2%) | 512 (23.6%) | |
| Hospital Location | ||||
| Community Hospital | 757 (37.8%) | 46 (27.5%) | 803 (37.0%) | |
| Endoscopy Center | 2 (0.1%) | 0 (0%) | Fisher p = 0.017 | 2 (0.1%) |
| Quaternary Care Facility | 1246 (62.1%) | 121 (72.5%) | 1367 (62.9%) | |
| Thirty-Day Mortality | ||||
| Yes | 49 (2.4%) | 5 (3.0%) | 54 (2.5%) | |
| No | 1956 (97.6%) | 162 (97.0%) | Fisher p = 0.604 | 2118 (97.5%) |
| Length of Stay (in days) | ||||
| Median [Q1, Q3] | 3.2 [1.9, 5.9] | 7.7 [4.2, 13.8] | Kruskal–Wallis test p < 0.001 | 3.23 [1.98, 6.43] |
| Before Overlap Weighting | After Overlap Weighting | |||||
|---|---|---|---|---|---|---|
| Variables | Control (N = 1981) * | PH (N = 164) * | SMD | Control (N = 1981) * | PH (N = 164) * | SMD |
| Age (years), mean (SD) | 57.4 (17.18) | 62.02 (13.72) | 0.29 | 62.62 (15.65) | 62.62 (14.10) | <0.01 |
| Gender | 0.08 | <0.01 | ||||
| Female | 1310 (66.1) | 102.0 (62.2) | 72.4 (60.8) | 72.4 (60.8) | ||
| Male | 671.0 (33.9) | 62.0 (37.8) | 46.6 (39.2) | 46.6 (39.2) | ||
| Race | 0.39 | <0.01 | ||||
| White, non-Hispanic | 1450 (73.2) | 91.0 (55.5) | 71.6 (60.1) | 71.6 (60.1) | ||
| Hispanic | 165 (8.3) | 18 (11.0) | 12.0 (10.1) | 12.0 (10.1) | ||
| Black | 317 (16.0) | 46.0 (28.0) | 30.1 (25.3) | 30.1 (25.3) | ||
| Other | 49.0 (2.5) | 9.0 (5.5) | 5.3 (4.5) | 5.3 (4.5) | ||
| BMI, mean (SD) | 34.2 (112.4) | 29.6 (8.8) | 0.06 | 29.6 (15.1) | 29.6 (9.0) | <0.01 |
| Number of Elixhauser comorbidities, mean (SD) | 3.2 (2.55) | 6.9 (1.97) | 1.64 | 6.5 (2.77) | 6.5 (1.83) | <0.01 |
| Hospital Location | 0.23 | <0.01 | ||||
| Community Hospital | 753 (38.0) | 45 (27.4) | 36.3 (30.5) | 36.3 (30.5) | ||
| Endoscopy Center | 2.0 (0.1) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | ||
| Quaternary Care Facility | 1226.0 (61.9) | 119 (72.6) | 82.7 (69.5) | 82.7 (69.5) | ||
| Procedure Severity | 0.80 | <0.01 | ||||
| Minor or Intermediate | 644 (32.5) | 112 (68.3) | 76 (63.9) | 76 (63.9) | ||
| Major | 852 (43.0) | 25 (15.2) | 21 (18.0) | 21 (18.0) | ||
| Xmajor/Complex | 485 (24.5) | 27 (16.5) | 21 (18.2) | 21 (18.2) | ||
| Outcome | Unadjusted * | Propensity Overlap-Weighted Model ** | ||||
|---|---|---|---|---|---|---|
| PH | Control | p-Value | RR | 95% CI | p-Value | |
| PPC | 49 (29.9%) | 221 (11.2%) | <0.001 | 1.66 | 1.05–2.71 | 0.036 |
| RF | 31(19.3%) | 131 (6.6%) | <0.001 | 1.68 | 0.90–3.28 | 0.109 |
| PNA | 18 (11.2%) | 113 (5.7%) | 0.010 | 1.21 | 0.57–2.65 | 0.615 |
| ASP | 6 (3.7%) | 34 (1.7%) | 0.118 (f) | 1.63 | 0.38–8.28 | 0.514 |
| PE | 5 (3.1%) | 30 (1.5%) | 0.181 (f) | 1.2 | 0.29–5.28 | 0.794 |
| 30-day mortality | 5 (3.0%) | 49 (2.5%) | 0.604 (f) | 1.23 | 0.43–2.76 | 0.651 |
| Length of stay, median days (IQR) | 8 (6.8, 9.1) | 4.9 (4.4, 5.2) | <0.001 | 0.63 | 0.53–0.77 | <0.001 |
| Variable | PH Group | Pre-Capillary | Post-Capillary | p-Value * |
|---|---|---|---|---|
| Age (years) | 63.2 (13.9) | 59.88 (14.8) | 63.0 (13.5) | 0.18 |
| BMI (kg/m2) | 29.6 (8.9) | 27.8 (10.4) | 30.2 (8.0) | 0.12 |
| Echocardiography | ||||
| Right Ventricular Systolic Pressure (mmHg) | 52.9 (20.6) | 54.3 (20.3) | 52.2 (20.9) | 0.59 |
| Right Heart Catheterization | ||||
| Mean Right Atrial Pressure (mmHg) | 13.2 (7.7) | 9.5 (5.9) | 14.6 (8.0) | <0.001 |
| Right Ventricular Systolic Pressure (mmHg) | 53.8 (18.9) | 51.9 (19.8) | 54.5 (18.6) | 0.42 |
| Pulmonary Artery Systolic Pressure (mmHg) | 53.3 (18.4) | 51.2 (19.9) | 54.0 (17.7) | 0.37 |
| Pulmonary Artery Diastolic Pressure (mmHg) | 25.0 (9.1) | 21.5 (8.6) | 26.4 (8.9) | 0.002 |
| Pulmonary Artery Mean Pressure (mmHg) | 35.7 (11.6) | 33.2 (11.4) | 36.7 (11.4) | 0.08 |
| Pulmonary Artery Occlusion Pressure (mmHg) | 19.1 (7.3) | 11.35 (2.5) | 22.2 (6.2) | <0.001 |
| Pulmonary Artery Oxygen Saturation (%) | 64.3 (9.6) | 65.5 (8.5) | 64.0 (9.8) | 0.39 |
| Fick Cardiac Output (L/min) | 5.9 (2.5) | 5.2 (1.9) | 6.2 (2.6) | 0.02 |
| Calculated Cardiac Index | 3.1 (1.2) | 3.0 (1.2) | 3.1 (1.1) | 0.60 |
| Primary and Secondary Outcomes ** | Pre-capillary (N = 47) | Post-capillary (N = 116) | p-value | |
| PPC | 15 (31.9%) | 34 (29.3%) | 0.889 | |
| RF | 10 (21.3%) | 21 (18.6%) | 0.863 | |
| PNA | 2 (4.3%) | 16 (14.2%) | 0.126 | |
| ASP | 3 (6.4%) | 3 (2.7%) | 0.36 | |
| PE | 1 (2.1%) | 4 (3.5%) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatsuoka, Y.; Carr, Z.J.; Jayakumar, S.; Lin, H.-M.; He, Z.; Farroukh, A.; Heerdt, P. Pulmonary Hypertension and the Risk of 30-Day Postoperative Pulmonary Complications after Gastrointestinal Surgical or Endoscopic Procedures: A Retrospective Propensity Score-Weighted Cohort Analysis. J. Clin. Med. 2024, 13, 1996. https://doi.org/10.3390/jcm13071996
Tatsuoka Y, Carr ZJ, Jayakumar S, Lin H-M, He Z, Farroukh A, Heerdt P. Pulmonary Hypertension and the Risk of 30-Day Postoperative Pulmonary Complications after Gastrointestinal Surgical or Endoscopic Procedures: A Retrospective Propensity Score-Weighted Cohort Analysis. Journal of Clinical Medicine. 2024; 13(7):1996. https://doi.org/10.3390/jcm13071996
Chicago/Turabian StyleTatsuoka, Yoshio, Zyad J. Carr, Sachidhanand Jayakumar, Hung-Mo Lin, Zili He, Adham Farroukh, and Paul Heerdt. 2024. "Pulmonary Hypertension and the Risk of 30-Day Postoperative Pulmonary Complications after Gastrointestinal Surgical or Endoscopic Procedures: A Retrospective Propensity Score-Weighted Cohort Analysis" Journal of Clinical Medicine 13, no. 7: 1996. https://doi.org/10.3390/jcm13071996