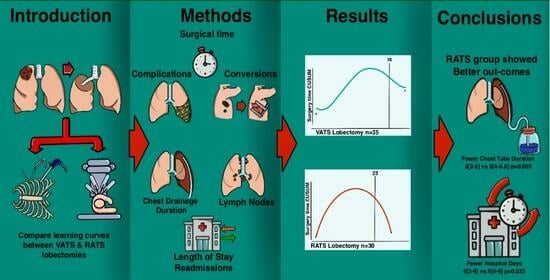
Robotic Lobectomy Learning Curve Has Better Clinical Outcomes than Videothoracoscopic Lobectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Type
2.2. Demographics and Patients’ Characteristics/Variables Used
2.3. Selection Criteria
2.4. Centrality
2.5. Surgeon’s Expertise and Training in VATS and RATS Anatomical Lung Resections
2.6. Surgical Technique
2.7. Surgical Time
2.8. Conversion
2.9. Chest Tube and Discharge
2.10. Statistical Analysis
3. Results
3.1. Patient Demographics and Characteristics
3.2. Learning Curve
3.3. Surgical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKenna, R.J. Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J. Thorac. Cardiovasc. Surg. 1994, 107, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Cao, C.; D’Amico, T.A.; Demmy, T.L.; He, J.; Hansen, H.; Swanson, S.J.; Walker, W.S.; Casali, G.; Dunning, J.; et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: A consensus statement. Eur. J. Cardio-Thoracic Surg. 2013, 45, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, D.; Orlowski, T. The Role of VATS in lung cancer surgery: Current status and prospects for development. Minim. Invasive Surg. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Lim, E.; Batchelor, T.J.; Dunning, J.; Shackcloth, M.; Anikin, V.; Naidu, B.; Belcher, E.; Loubani, M.; Zamvar, V.; Harris, R.A.; et al. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid. 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Melfi, F.M.; Menconi, G.F.; Mariani, A.M.; Angeletti, C.A. Early experience with robotic technology for thoracoscopic surgery. Eur. J. Cardio-Thorac. Surg. 2002, 21, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Farivar, A.S.; Cerfolio, R.J.; Vallières, E.; Knight, A.W.; Bryant, A.; Lingala, V.; Aye, R.W.; Louie, B.E. Comparing Robotic Lung Resection with Thoracotomy and Video-Assisted Thoracoscopic Surgery Cases Entered into the Society of Thoracic Surgeons Database. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2014, 9, 10–15. [Google Scholar] [CrossRef]
- Veronesi, G.; Novellis, P.; Voulaz, E.; Alloisio, M. Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 2016, 101, 28–34. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bess, K.M.; Wei, B.; Minnich, D.J. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann. Thorac. Surg. 2016, 102, 394–399. [Google Scholar] [CrossRef]
- Singer, E.; Kneuertz, P.J.; D’souza, D.M.; Moffatt-Bruce, S.D.; Merritt, R.E. Understanding the financial cost of robotic lobectomy: Calculating the value of innovation? Ann. Cardiothorac. Surg. 2019, 8, 194–201. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Brunelli, A.; Kim, A.W.; Berger, K.I.; Addrizzo-Harris, D.J. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e166S–e190S. [Google Scholar] [CrossRef]
- Boada, M.; Guzmán, R.; Montesinos, M.; Libreros, A.; Guirao, A.; Sánchez-Lorente, D.; Gimferrer, J.; Agustí, A.; Molins, L. Upstaging, Centrality and Survival in Early Stage Non-Small Cell Lung Cancer Video-Assisted Surgery. Lung Cancer 2019, 134, 254–258. [Google Scholar] [CrossRef]
- Paglialunga, P.L.; Molins, L.; Guzmán, R.; Guirao, A.; Grando, L.; Sanchez-Lorente, D.; Guerrero, C.; Bello, I.; Quiroga, N.; Boada, M. Starting a robotic thoracic surgery program: From wedge resection to sleeve lobectomy in six months. Initial conclusions. Cirugía Española 2023, 101, 833–840. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Wittekind, C.; Goldstraw, P. Complete resection in lung cancer surgery: Proposed definition. Lung Cancer 2005, 49, 25–33. [Google Scholar] [CrossRef]
- Lee, B.E.; Shapiro, M.; Rutledge, J.R.; Korst, R.J. Nodal Upstaging in Robotic and Video Assisted Thoracic Surgery Lobectomy for Clinical N0 Lung Cancer. Ann. Thorac. Surg. 2015, 100, 229–234. [Google Scholar] [CrossRef]
- Ureña, A.; Moreno, C.; Macia, I.; Rivas, F.; Déniz, C.; Muñoz, A.; Serratosa, I.; García, M.; Masuet-Aumatell, C.; Escobar, I.; et al. A Comparison of Total Thoracoscopic and Robotic Surgery for Lung Cancer Lymphadenectomy. Cancers 2023, 15, 3442. [Google Scholar] [CrossRef]
- Jang, H.-J.; Lee, H.-S.; Park, Y.; Zo, J.I. Comparison of the Early Robot-Assisted Lobectomy Experience to Video-Assisted Thoracic Surgery Lobectomy for Lung Cancer A Single-Institution Case Series Matching Study. Innovations 2011, 6, 305–310. [Google Scholar] [CrossRef]
- Augustin, F.; Bodner, J.; Maier, H.; Schwinghammer, C.; Pichler, B.; Lucciarini, P.; Pratschke, J.; Schmid, T. Robotic-assisted minimally invasive vs. thoracoscopic lung lobectomy: Comparison of perioperative results in a learning curve setting. Langenbeck’s Arch. Surg. 2013, 398, 895–901. [Google Scholar] [CrossRef]
- Rinieri, P.; Bubenheim, M.; Calenda, E.; Melki, J.; Peillon, C.; Baste, J.-M.; Mahieu, J. Robot-Assisted Thoracoscopic Surgery versus Video-Assisted Thoracoscopic Surgery for Lung Lobectomy: Can a Robotic Approach Improve Short-Term Outcomes and Operative Safety? Thorac. Cardiovasc. Surg. 2015, 64, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Louie, B.E.; Farivar, A.S.; Aye, R.W.; Vallières, E. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann. Thorac. Surg. 2012, 93, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.S.; Hartwig, M.G.; Vallières, E.; Abbas, A.E.; Cerfolio, R.J.; Dylewski, M.R.; Fabian, T.; Herrera, L.J.; Jett, K.G.; Lazzaro, R.S.; et al. Pulmonary Open, Robotic, and Thoracoscopic Lobectomy (PORTaL) Study: An Analysis of 5721 Cases. Ann. Surg. 2021, 277, 528–533. [Google Scholar] [CrossRef]
- Mazzella, A.; Olland, A.; Falcoz, P.E.; Renaud, S.; Santelmo, N.; Massard, G. Video-assisted thoracoscopic lobectomy: Which is the learning curve of an experienced consultant? J. Thorac. Dis. 2016, 8, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Arnold, B.N.; Thomas, D.C.; Bhatnagar, V.; Blasberg, J.D.; Wang, Z.; Boffa, D.J.; Detterbeck, F.C.; Kim, A.W. Defining the learning curve in robot-assisted thoracoscopic lobectomy. Surgery 2019, 165, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Sun, X.; Miao, S.; Li, S.; Zhao, Y.; Xuan, Y.; Qiu, T.; Niu, Z.; Song, J.; Jiao, W. Learning curve for robot-assisted lobectomy of lung cancer. J. Thorac. Dis. 2019, 11, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Özyurtkan, M.O.; Kaba, E.; Ayalp, K.; Demirhan, Ö.; Uyumaz, E. Robotic anatomic lung resections: The initial experience and description of learning in 102 cases. Surg. Endosc. 2015, 30, 676–683. [Google Scholar] [CrossRef]
- Meyer, M.; Gharagozloo, F.; Tempesta, B.; Margolis, M.; Strother, E.; Christenson, D. The learning curve of robotic lobectomy. Int. J. Med. Robot. Comput. Assist. Surg. 2012, 8, 448–452. [Google Scholar] [CrossRef]
- Mazzella, A.; Mohamed, S.; Maisonneuve, P.; Sedda, G.; Cara, A.; Casiraghi, M.; Petrella, F.; Donghi, S.M.; Iacono, G.L.; Spaggiari, L. Learning Curve of Robotic Lobectomy for the Treatment of Lung Cancer: How Does It Impact on the Autonomic Nervous System of the Surgeon? J. Pers. Med. 2023, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Han, Y.; Xiang, J.; Cerfolio, R.J.; Li, H. Robotic Anatomical Segmentectomy: An Analysis of the Learning Curve. Ann. Thorac. Surg. 2019, 107, 1515–1522. [Google Scholar] [CrossRef]
- Fortea-Sanchis, C.; Escrig-Sos, J. Quality control techniques in surgery: Application of cumulative sum (CUSUM) charts. Cirugía Española 2019, 97, 65–70. [Google Scholar] [CrossRef]
- Geraci, T.C.; Chang, S.H.; Chen, S.; Ferrari-Light, D.; Cerfolio, R.J. Discharging Patients by Postoperative Day One After Robotic Anatomic Pulmonary Resection. Ann. Thorac. Surg. 2022, 114, 234–240. [Google Scholar] [CrossRef]
- Harrison, O.J.; Maraschi, A.; Routledge, T.; Lampridis, S.; LeReun, C.; Bille, A. A cost analysis of robotic vs. video-assisted thoracic surgery: The impact of the learning curve and the COVID-19 pandemic. Front. Surg. 2023, 10, 1123329. [Google Scholar] [CrossRef] [PubMed]






| RATS (n = 30) | VATS (n = 35) | p Value | |
|---|---|---|---|
| Age * years | 65.69 (9.85) | 70.41 (9.58) | 0.059 |
| Gender; n (%) | 0.134 | ||
| Female | 16 (53.3) | 12 (34.3) | |
| Male | 14 (46.7) | 23 (65.7) | |
| Smoker; n (%) | 0.917 | ||
| Active | 11 (37.9) | 11 (32.4) | |
| Former | 6 (20.7) | 8 (23.5) | |
| Never | 11 (34.5) | 12 (32.4) | |
| Unknown | 2 (6.9) | 4 (11.8) | |
| Comorbidity_yes; n (%) | 18 (60) | 22 (62.8) | 1 |
| COPD; n (%) | 5 (16.6) | 7 (20) | 1 |
| HT; n (%) | 16 (53.3) | 22 (62.8) | 0.435 |
| DM; n (% | 5 (16.6) | 10 (28.5) | 0.375 |
| CRI; n (%) | 4 (13.3) | 3 (8.5) | 0.694 |
| CV; n (%) | 9 (30) | 12 (34.2) | 0.793 |
| Anticoagulant; n (%) | 6 (20) | 6 (17.1) | 1 |
| FEV1_L * | 2.29 (0.69) | 2.35 (0.74) | 0.756 |
| FEV1_% * | 88 (17.87) | 78.59 (16.78) | 0.035 |
| FVC_L † | 3.20 (2.70, 4.09) | 3.09 (2.66, 3.99) | 0.847 |
| FVC_% * | 93.34 (15.81) | 86.88 (15.68) | 0.109 |
| DLCO% † | 83 (77, 90) | 77 (68, 91) | 0.408 |
| Tumor location; n (%) | 0.694 | ||
| Right upper lobectomy | 11 (36.6%) | 13 (37.1%) | |
| Right middle lobectomy | 2 (6.6%) | 1 (2.85%) | |
| Right lower lobectomy | 5 (16.6%) | 10 (28.5%) | |
| Left upper lobectomy | 7 (23.3%) | 8 (22.8%) | |
| Left lower lobectomy | 5 (16.6%) | 3 (8.57%) | |
| Tumor size; cm (SD) | 1.70 (1.30, 2.50) | 2.30 (1.33, 3.00) | 0.307 |
| Preoperative induction treatment; n | 0 (0%) | 1 (2.85%) | 1 |
| Centrality; n | 2 (6.6%) | 2 (5.7%) | 1 |
| Number of LNs; n (SD) | 9 (7, 12.8) | 9 (7, 12.8) | 0.738 |
| Sampled LNs stations; n (SD) | 5 (4, 5) | 4 (4, 5) | 0.174 |
| Superior mediastinal LN; n (SD) | 2 (0, 3) | 1 (0, 4) | 0.690 |
| Station 7; n (SD) | 3 (2, 3.8) | 2 (1.2, 3) | 0.189 |
| Mediastinal LNs; n (SD) | 0 (0, 0.8) | 0 (0, 1) | 0.273 |
| Inferior mediastinal LNs; n (SD) | 1 (0, 1.8) | 1 (0, 2) | 0.814 |
| Pulmonary hilar LNs; n (SD) | 1 (1, 2) | 1 (0, 3.5) | 0.773 |
| pStage TNM 8th | 1 | ||
| I; n | 20 (76.9%) | 25 (73.5%) | |
| II; n | 5 (19.2%) | 7 (20.6%) | |
| III; n | 1 (3.8%) | 2 (5.9%) |
| RATS (n = 30) | VATS (n = 35) | p Value | |
|---|---|---|---|
| Operative time; minutes (range) | 204 (165–230) | 190 (180–210) | 0.772 |
| Learning curve completion; n | 23 | 28 | |
| Adjusted learning curve completion; n | 23 | 31 | |
| Surgical failure; n (%) | 8 (26.6%) | 14 (40%) | 0.292 |
| Conversion; n (%), causes | 1 (3.3%) Oncologic causes | 1 (2.8%) Firm adhesions | |
| Complications; n (%) | 4 (13.3%) | 8 (22.8%) | 0.358 |
| Grade I; n (%) | 3 grade I (persistent air leaks) | 5 grade I (air leaks) | |
| Grade II; n (%) | 0 | 3 grade II (Anaphylactic shock, chylothorax, bradycardia with heart block) | |
| Grade III; n (%) | 1 grade III (bleeding requiring surgery) | 0 | |
| Grade IV; n (%) | 0 | 0 | |
| Readmission; n (%) | 4 (13.3%) | 8 (22.8%) | 0.358 |
| Readmission causes | Difficult pain control Upper Gastrointestinal Bleeding, Fever Wound Infection | Fever in two cases Cardiovascular event, septic shock, pain, respiratory infection hemoptysis | |
| Days of chest drainage; days (range) | 3 (2–5) | 5 (4–5.8) | 0.005 |
| Length of stay; days (range) | 4 (3–6) | 5 (4–6) | 0.023 |
| 90-day mortality; n | 0 | 0 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paglialunga, P.L.; Molins, L.; Guzmán, R.; Guirao, A.; Bello, I.; Ureña, A.; Grando, L.; Quiroga, N.; Michavila, X.; Boada, M. Robotic Lobectomy Learning Curve Has Better Clinical Outcomes than Videothoracoscopic Lobectomy. J. Clin. Med. 2024, 13, 1653. https://doi.org/10.3390/jcm13061653
Paglialunga PL, Molins L, Guzmán R, Guirao A, Bello I, Ureña A, Grando L, Quiroga N, Michavila X, Boada M. Robotic Lobectomy Learning Curve Has Better Clinical Outcomes than Videothoracoscopic Lobectomy. Journal of Clinical Medicine. 2024; 13(6):1653. https://doi.org/10.3390/jcm13061653
Chicago/Turabian StylePaglialunga, Pablo Luis, Laureano Molins, Rudith Guzmán, Angela Guirao, Irene Bello, Anna Ureña, Leandro Grando, Nestor Quiroga, Xavier Michavila, and Marc Boada. 2024. "Robotic Lobectomy Learning Curve Has Better Clinical Outcomes than Videothoracoscopic Lobectomy" Journal of Clinical Medicine 13, no. 6: 1653. https://doi.org/10.3390/jcm13061653