2. Materials and Methods
2.1. Study Design and Patient Selection
As a spontaneous breathing anesthetic approach, the SVI method is a novel alternative to previously reported techniques. In our case series, we applied the SVI method in a prospective, nonconsequential manner when patients met the inclusion and exclusion criteria (
Table 1), and a dedicated anesthetist was assigned. Retrospective data including the intraoperative and early postoperative periods were collected and statistically analyzed to assess the feasibility of the SVI method as a primary endpoint and to identify any potential limiting factors. All patients were informed about the risks and benefits of the SVI method compared to classic anesthetic management before the operation.
This study was approved by the Ethical Committee of the Human Investigation Review Board at the University of Szeged (permission no.:4703/2020.01.20, Chairperson Professor Tibor Wittman, address: Koranyi fasor 8–10, Szeged, Hungary).
For patient selection, we applied our previously published criteria for non-intubated thoracic procedures (NITS) (
Table 1) [
15]. However, the contraindications for NITS do not exclude the use of the SVI method [
16]. In our daily clinical setting, patients with a body mass index (BMI) < 34 without other contraindications were deemed suitable for SVI.
Preoperative pulmonological and anesthesiologic examinations were the same as those in the normal or NITS cases. From a surgical perspective, we included all cases of SVI that would also be suitable for normal VATS according to consensus meeting recommendations. We included patients with no advanced lung cancer (<7 cm, N0, or N1) [
17].
Between 10 March 2020 and 28 October 2022, 141 surgeries were performed by our thoracic surgery team using the SVI approach for general anesthesia. All surgeries were performed by a single surgeon, and the patients were anesthetized by three anesthetists using the same procedural algorithm. Initially, we intended to perform 144 SVI procedures. Three cases were excluded from statistical analyses because spontaneous breathing did not return by the end of the surgery.
2.2. Anesthetic Management
Three-lead ECG, oxygen saturation (SpO2) and invasive blood pressure measurements were performed. The depth of anesthesia was monitored using the bispectral index (BIS, Medtronic Vista). Anesthesia was induced with fentanyl (1–1.5 µg/kg) and propofol using target-controlled infusion (Schnider model) with effect-site targeting. Considering the induction effect, the site target concentration was generally set between 4 and 6 µg/mL, depending on individual patient characteristics. Subsequently, the target concentration was modified to keep the BIS value between 40 and 60.
Mivacurium chloride (0.1–0.15 µg/kg), a short-acting non-depolarizing muscle relaxant, was used to ensure optimal conditions for intubation. Although the dose of mivacurium that was used was below the recommended dose for intubation, we found that, 180 s after drug administration, the conditions for intubation were good or excellent.
Similar to the gold standard approach, fiberoptic equipment (aScope, Ambu, Ballerup, Denmark) was used to confirm the proper position of the DLT. Confirmation of the proper tube position was crucial because further manipulation of the tube after the effects of the muscle relaxant diminished would not be well tolerated.
Muscle relaxation facilitated the DLT insertion and helped in early surgical steps. After the thoracic cavity was opened, a paravertebral nerve blockade for pain relief and a vagal blockade to prevent the cough reflex were performed. Spontaneous breathing returned after the muscle relaxant effect was eliminated. In unexpected cases, when spontaneous breathing was unsatisfactory (low tidal volumes, bradypnea), temporary pressure support ventilation with a low-flow trigger (1.0 L/min) was used until the muscle relaxant effect was fully eliminated.
Intraoperatively, altering the FiO2 (40–100%) and applying 3–5 H2O cm positive end-expiratory pressure (PEEP) to the dependent lung helped keep the SpO2 and PaCO2 within normal or close-to-normal ranges. Severe hypercapnia or hypoxia was also prevented or managed by applying pressure support ventilation to the dependent lung.
In case of necessity, the intraoperative evaluation of air leakage involved conducting a water submersion test (WST). After filling the thoracic cavity with saline, manual positive pressure ventilation was synchronized with the patient’s spontaneous breathing activity. Furthermore, if it was necessary, we were able to apply pressure support ventilation to perform the leak test.
2.3. Rescue Maneuvers
2.3.1. Hypotension
According to our intraoperative hemodynamic management protocol, when the mean arterial pressure was <60 mmHg, the systolic blood pressure was <90 mmHg or decreased by more than 25%, and ephedrin (5–10 mg) or phenylephrine (50–100 µg) was administered in divided doses.
2.3.2. Hypoxia/Hypercapnia
In patients with SpO
2 < 92% or PaO
2 < 60 mmHg, 3–5 H
2O cm PEEP administration and FiO
2 alteration were used to increase oxygenation. In the cases of PaCO
2 > 75 mmHg or pH < 7.15, the non-dependent lung was considered to be re-inflated for a short period to eliminate carbon dioxide and improve oxygenation. The effect of reinflation is partial and temporary. Thus, if the improvement was not satisfactory, or as an initial step, we administered pressure support ventilation with 3–5 H
2O cm PEEP and 8–14 H
2O cm pressure support to assist in gas exchange. If hypercapnia and/or hypoxia were persistent, anesthetic conversion with muscle relaxation (0.05–0.1 µg/kg of mivacurium) and volume-controlled mechanical ventilation (PEEP: 3–5 H
2O cm, tidal volume: 3–4 mL/kg, Pmax < 30 H
2O cm) were applied (
Figure 1).
2.3.3. Technical Difficulties
Paradoxical mediastinal shifting is an accompanying phenomenon of spontaneous ventilation surgery. The mediastinum moves downward during inspiration and vice versa during expiration. If it was intolerable, pressure support ventilation was applied to overcome the issue. An ineffective vagal nerve blockade may result in coughing. If repeated vagal nerve infiltration was not feasible, anesthetic conversion was applied (
Figure 1).
2.4. Regional Anesthetic Techniques
In our study, all regional anesthetic techniques were performed by the surgeon under direct vision to decrease the risk of complications associated with a regional blockade and to reduce the length of stay in the operating room. During VATS with SVI in routine cases, 5 mg/kg of lidocaine (2%) was administered at the site of incision at the fifth intercostal space in the mid-axillary line. After opening the thoracic cavity under thoracoscopic guidance, vagal and paravertebral blocks were performed. For the vagal nerve blockade, 3–5 mL of bupivacaine (0.5%) was administered close to the nerve (aortopulmonary window, left side; upper mediastinum, right side). A deep intercostal or paravertebral blockade was achieved by administering 4–5 mL of bupivacaine (0.5%) close to each intercostal nerve (from the second to the fifth intercostal space). The maximum amount of bupivacaine used was 0.5 mL/kg (2.5 mg/kg). In cases of open SVI, an intercostal nerve blockade was guaranteed by administering 4–5 mL of bupivacaine (0.5%) between the third and sixth intercostal spaces.
2.5. Surgical Technique
We performed the same VATS uniportal method during the SVI procedures that we published in our NITS study [
15,
18], with indications based on the European Society of Thoracic Surgeons consensus report [
19] and the recommendation of the NITS pioneers [
20,
21].
2.6. Postoperative Care
Every patient was observed in the post-anesthesia care unit (PACU) for at least 2 h or until they met the criteria for leaving the PACU (visual analog scale [VAS] score < 3, Aldrete score > 9). Oxygen was administered to all patients via a face mask with 4–6 L/min O2 postoperatively to achieve a SpO2 of >94% or >88% in patients with chronic obstructive pulmonary disease. None of the patients required a higher level of oxygen or a higher degree of respiratory support (non-invasive ventilation) during the PACU stay or in the later postoperative period. None of the patients experienced fever or required bronchial secretion removal. Postoperatively, chest radiography was performed before and after the chest tube removal. Any pneumothorax, atelectasia/dystelectasia, infiltration or pleural fluid observed in the radiography were considered abnormal findings. The pain intensity during hospitalization was assessed using a numeric pain rating scale (NPRS). An NPRS > 3 was the intervention point, and a minor analgesic agent prescribed by the anesthetist was administered orally as soon as possible. For patients who underwent thoracotomy, a pleural catheter was inserted for continuous local anesthetic (0.1 mL/h/kgbw bupivacain 0.33%) administration.
2.7. Arterial Blood Sampling
In cases of major pulmonary resection, blood samples were collected four times (T1, T2, T3 and T4;
Figure 2). For T1, preoperative blood samples were collected before anesthesia induction, with a FiO
2 of 0.21. For T2, steady-state blood samples were collected 15 min after the vagal nerve blockade. For T3, blood samples were collected 15 min after anatomical resection (and only during anatomical resections). For T4, postoperative blood samples were collected 30 min after the patient arrived in the recovery room and at a FiO
2 of 0.5.
2.8. Data Collection and Analyses
Data were retrospectively collected from our medical system (e-MedSolution) and personal patient documentation. Personal patient data were also collected. Descriptive statistics were performed using R statistical software, version 4.2.2 (R Foundation, Vienna, Austria), and SPSS for Windows, version 26.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics are presented as the mean ± standard deviation (SD) for continuous variables and as the count and percentage for categorical variables.
4. Discussion
The main concern with the non-intubated technique is airway safety. Without lung isolation, CO
2 rebreathing (the pendelluft phenomenon) is a possible consequence of chest opening. Hypoxia and hypercapnia can develop as a result of pendelluft and paradoxical mediastinal shifting, which are the main obstacles to the widespread acceptance of NITS among anesthetists and surgeons [
22,
23,
24,
25]. In our practice, as published earlier by our workgroup [
15], the non-intubated anesthetic approach encompasses BIS-guided propofol TCI with fentanyl administration for the induction of anesthesia, followed by laryngeal mask insertion. Therefore, the most relevant difference between SVI and NITS from our perspective is the question of airway safety. However, other workgroups (and their patients) may profit from general anesthesia itself.
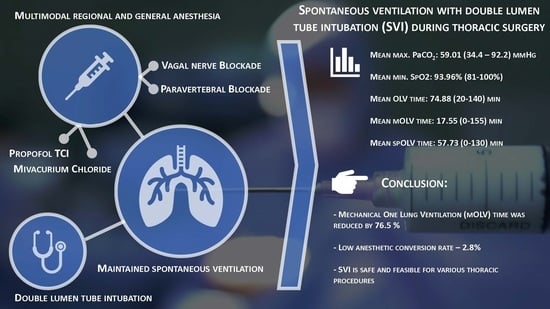
SVI combines the beneficial effects of spontaneous ventilation thoracic procedures with airway safety via tracheal intubation using a double-lumen tube. The essential components of SVI are double-lumen tube intubation for maximal airway safety, short-acting non-depolarizing muscle relaxants for the early recovery of spontaneous ventilation, paravertebral blockades as part of multimodal pain management and vagal nerve blockades to prevent the cough reflex. Infiltration of the vagal nerve with 2–5 mL of bupivacain (0.25–0.5%) into the thoracic cavity is a widely accepted method [
2,
12,
15,
26,
27], and repeated vagal nerve blockades may be necessary [
26]. However, in our practice, a single infiltration was sufficient for a mean of 80.2 min (25–150 min). From the perspective of the anesthetist, the initial stage of SVI resembles that of conventional thoracic procedures. After cessation of the muscle relaxant, patients breathed spontaneously without coughing. In three cases, spontaneous breathing was not detected until the end of surgery, despite the reduced dose of mivacurim, although all patients underwent short procedures. Furthermore, anesthetic management was similar to that of the NITS procedures. From the surgeon’s perspective, there was no major difference between SVI and NITS.
Double-lumen endotracheal tube placement provides an opportunity for all anesthetic interventions (recruitment maneuvers and fiberoptic control) with complete lung isolation. Therefore, if a surgical or anesthetic complication occurs, conversion to the conventional anesthetic pathway is easy and safe. The most risky part of the NITS procedure is the conversion process under aggravated circumstances (2–11% of cases) [
12,
27,
28,
29,
30,
31], which is prevented. Although single-lumen intubation with a blocker may be considered less invasive, a bronchial blocker is used as a secondary option in our institute for situations in which the placement of the DLT is not possible. When explained with the potential advantages, DLTs offer greater stability and are less prone to dislodgment during surgery, as well as more effective lung isolation. Furthermore, it is essential to consider the cost-effectiveness of each approach and the available resources.
Furthermore, the incidence of unforeseen difficult airways and difficult intubation can reach up to 20%. According to Langiano et al., the overall incidence of difficult airways is 16% in the thoracic surgical patient population, whereas the frequency of unexpected difficult airways is 5.2% [
32]. Corso et al. reported in a retrospective study of 763 patients that difficult intubation occurred in 13.6% of cases, challenging mask ventilation occurred in 9%, and a combination of both difficulties occurred in 2% [
33]. In such instances, SVI may provide a secure procedure for spontaneous ventilation. Moreover, when the indication for conversion is surgical (thoracotomy), muscle relaxation and controlled mechanical ventilation are not required. Spontaneous ventilation surgeries require surgeons to leave their comfort zone because paradoxical mediastinal shifting and diaphragmatic movements create an unusual surgical field. In their meta-analysis, Shi et al. reported that mediastinal and diaphragmatic factors were the most common complications leading to anesthetic conversion (7% and 4%, respectively) [
34]. In our SVI case series, muscle relaxation and permanently controlled mechanical OLV occurred in one patient due to intolerable mediastinal shifting (1/141, 0.71%). However, the incidence of disturbed mediastinal movement was higher but was managed with pressure support ventilation. Two of our anesthetic conversions (2/141, 1.42%) were due to ineffective vagal nerve blockades, and repeated vagal nerve infiltration was technically infeasible. The fourth anesthetic conversion was due to DLT dislodgement (1/141, 0.71%). The remaining 97.16% of patients did not require further muscle relaxation after the initial dose of the muscle relaxant for induction. Twelve surgical conversions from VATS SVI to open SVI were uneventful and did not require anesthetic conversion.
Gas exchange imbalance is common during spontaneous ventilation surgery. Hypoxia and hypercapnia have also been observed during NITS [
34]. In SVI, hypoxia is easily compensated by a higher FiO
2, PEEP administration or applying pressure support. However, it is important to emphasize that the peak airway pressure is lower during spontaneous ventilation, with or without pressure support, than that during controlled ventilation [
16]. Hypercapnia is a multifactorial condition. However, deepening the anesthesia negatively affects respiratory activity. In contrast, mediastinal shifting reduces the tidal volume and lung compliance, and airway resistance is also increased by using a double-lumen tube. To overcome hypercapnia, pressure support ventilation is an intermediate step before anesthetic conversion to maintain spontaneous breathing. Hypercapnia and associated respiratory acidosis tend to be temporary, and the acid–base aberration is generally spontaneously corrected after the operated lung is reinflated [
22,
24,
35,
36]. We identified the lowest maximum breathing frequency of 6, which did not result in hypoxia or hypercapnia. This was attributed to the thymectomy performed, the patient’s overall good health, young age (36 years) and low BMI (19.1). According to our blood gas results taken 30 min after extubation (T4), the mean PaCO
2 was 47.44 mmHg (36.7–66.7 mmHg), and the pH was 7.332 (7.275–7.401). However, hypercapnia itself may result in better ventilation–perfusion matching and reduce intraoperative lung injury by suppressing inflammatory responses [
37,
38]. Furák et al. reasoned that SVI is more physiological in relation to gas exchange, and the authors found that a significantly lower lowest oxygen saturation and higher maximum PaCO
2 level were found in the non-intubated group (vs. the SVI group) [
39].
In our SVI series, hypotension commonly occurred after anesthesia induction and vagal nerve blockades. In total, 46.1% of our patients needed temporary pharmacological hemodynamic support. For the rest of the patients (53.9%), reductions in blood pressure were below our hemodynamic management cut-off value, and thus, self-regulation was sufficient to normalize blood pressure. The incidence of hypotension and the extent of intraoperative blood pressure reduction were higher among patients in the cardiovascular disease (CV) group. The elevated occurrence of hypotension can be attributed to the medications routinely used by patients in the CV group. Among these patients, 52 (52/95, 54.74%) were taking beta-blockers, 75 (75/95, 78.95%) were on antihypertensive medications (ACE inhibitors, ARBs, Ca channel blockers, imidazoline/α-2 receptor agonists), 30 (30/75, 40%) were on combinations of multiple antihypertensive drugs, and 15 patients (15/95, 15.79%) were regularly taking antidiuretic agents. As described earlier, SVI shows better hemodynamic stability than that of the non-intubated technique [
39]. The negative effects of controlled mechanical ventilation (CMV) have been extensively explored. Thus, the decrease in the mechanical OLV time (76.5%) suggests that our patients suffered from less oxidative stress, which may offer some immunological advantages [
6,
8,
9,
11].
Furák and Szabó previously published their results on SVI [
16] and SVI lobectomies [
39]. Their mean surgical time was 83.3 min (55–130 min) and 88.1 min (55–120 min), respectively. These times are similar to our mean surgical time of 80.2 min (25–150 min) and shorter than that reported in AlGhamdi’s study on non-intubated and intubated/relaxed VATS lobectomies (130.9, and 146.0 min) [
40]. Furthermore, Moon et al., in their study of 115 non-intubated thoracoscopic surgeries, reported that the mean operation time was 130 min [
31]. Similarly, Hung et al., in their study of 109 non-intubated thoracic procedures, reported a mean operative time of 124.4 min [
41]. Although the focus of this study is not the detailed surgical results, by comparing Furak’s first SVI study with AlGhamdi’s study, it can be found that the mean postoperative length of hospital stay after VATS SVI lobectomies and open SVI lobectomies was shorter than the length of stay reported in AlGhamdi’s study after non-intubated and intubated/relaxed VATS lobectomies (3.7 and 4.8 days for SVI VATS/open lobectomies and 6.9 and 7.6 days for non-intubated and intubated/relaxed lobectomies) [
16,
40]. Our conversion rate from VATS to open thoracotomy was 8.7%. All conversions were due to technical oncological reasons or due to bleeding, which was not associated with the SVI method. According to Power et al.’s systematic review and meta-analysis, based on the results of 72.932 patients, the median conversion rate from VATS to thoracotomy for anatomical resections was 9.6% [
42].
There are only a few established exclusion criteria for SVI, and patients indicated for VATS are also candidates for SVI. NITS has several exclusion criteria, such as potentially difficult airways or intubation, coagulation disorders or mental conditions, which are not exclusion reasons for SVI [
7,
40,
43]. In our case series, the contraindications for SVI were a high BMI, patient refusal, elevated intracranial pressure, hemodynamic instability and right heart failure (
Table 1). We accepted a higher threshold BMI as a general exclusion criterion for SVI (as well as for NITS) compared to other working groups because our national average BMI was slightly higher. Based on individual considerations, we sometimes made further concessions regarding the BMI if it was deemed justified by the patient’s condition and respiratory function.
Limitations
Our study has several limitations. More detailed perioperative monitoring (respiratory function tests, blood gas analyses and laboratory tests) and postoperative outpatient follow-ups would provide more comprehensive physiological and pathophysiological data related to SVI. Having compared the conventional and non-intubated methods, our findings suggest that future investigations are required to further evaluate the advantages and disadvantages of the SVI method.