Embryo Transfer Procedural Parameters Do Not Predict IVF Cycle Outcome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Controlled Ovarian Stimulation
2.2. Embryo Culture and Assessment
2.3. Embryo Transfer Procedure
2.4. Outcome Measures
2.5. Statistical Analyses
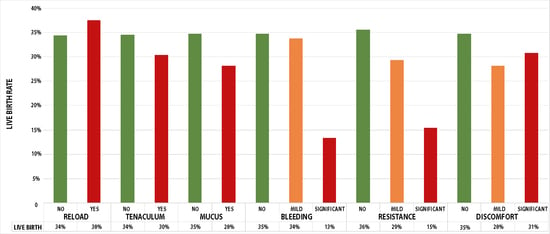
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, A.; Laufer, N. Assessment and Treatment of Repeated Implantation Failure (RIF). J. Assist. Reprod. Genet. 2012, 29, 1227–1239. [Google Scholar] [CrossRef]
- Schoolcraft, W.B. Importance of Embryo Transfer Technique in Maximizing Assisted Reproductive Outcomes. Fertil. Steril. 2016, 105, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Lensen, S.; Lantsberg, D.; Gardner, D.K.; Sophian, A.D.; Wandafiana, N.; Kamath, M.S. The Role of Timing in Frozen Embryo Transfer. Fertil. Steril. 2022, 118, 832–838. [Google Scholar] [CrossRef]
- Glujovsky, D.; Quinteiro Retamar, A.; Alvarez Sedo, C.; Ciapponi, A.; Cornelisse, S.; Blake, D. Cleavage-stage versus Blastocyst-stage Embryo Transfer in Assisted Reproductive Technology. Cochrane Database Syst. Rev. 2022, 5, CD002118. [Google Scholar] [CrossRef] [PubMed]
- Martins, W.P.; Nastri, C.O.; Rienzi, L.; van der Poel, S.Z.; Gracia, C.; Racowsky, C. Blastocyst vs Cleavage-Stage Embryo Transfer: Systematic Review and Meta-Analysis of Reproductive Outcomes. Ultrasound Obstet. Gynecol. 2017, 49, 583–591. [Google Scholar] [CrossRef]
- Maziotis, E.; Kalampokas, T.; Giannelou, P.; Grigoriadis, S.; Rapani, A.; Anifantakis, M.; Kotsifaki, A.; Pantou, A.; Triantafyllidou, O.; Tzanakaki, D.; et al. Commercially Available Molecular Approaches to Evaluate Endometrial Receptivity: A Systematic Review and Critical Analysis of the Literature. Diagnostics 2022, 12, 2611. [Google Scholar] [CrossRef]
- Nastri, C.O.; Martins, W.P. Ultrasound Guidance for Embryo Transfer: Where Do We Stand? Ultrasound Obstet. Gynecol. 2016, 48, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Penzias, A.; Bendikson, K.; Butts, S.; Coutifaris, C.; Falcone, T.; Fossum, G.; Gitlin, S.; Gracia, C.; Hansen, K.; La Barbera, A.; et al. Performing the Embryo Transfer: A Guideline. Fertil. Steril. 2017, 107, 882–896. [Google Scholar] [CrossRef]
- Tiras, B.; Korucuoglu, U.; Polat, M.; Saltik, A.; Zeyneloglu, H.B.; Yarali, H. Effect of Blood and Mucus on the Success Rates of Embryo Transfers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 239–242. [Google Scholar] [CrossRef]
- Arora, P.; Mishra, V. Difficult Embryo Transfer: A Systematic Review. J. Hum. Reprod. Sci. 2018, 11, 229–235. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Ragab, A.E.; Alboghdady, L.A.; Helal, A.S.; Bedairy, M.H.; Bahlol, I.A.; Abdelaziz, A. Difficult Embryo Transfer (ET) Components and Cycle Outcome. Which Is More Harmful? Middle East Fertil. Soc. J. 2016, 21, 114–119. [Google Scholar] [CrossRef]
- Phillips, J.A.S.; Martins, W.P.; Nastri, C.O.; Raine-Fenning, N.J. Difficult Embryo Transfers or Blood on Catheter and Assisted Reproductive Outcomes: A Systematic Review and Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Plowden, T.C.; Hill, M.J.; Miles, S.M.; Hoyt, B.; Yauger, B.; Segars, J.H.; Csokmay, J.M.; Chason, R.J. Does the Presence of Blood in the Catheter or the Degree of Difficulty of Embryo Transfer Affect Live Birth? Reprod. Sci. 2017, 24, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Kava-Braverman, A.; Martínez, F.; Rodríguez, I.; Álvarez, M.; Barri, P.N.; Coroleu, B. What Is a Difficult Transfer? Analysis of 7,714 Embryo Transfers: The Impact of Maneuvers during Embryo Transfers on Pregnancy Rate and a Proposal of Objective Assessment. Fertil. Steril. 2017, 107, 657–663.e1. [Google Scholar] [CrossRef] [PubMed]
- Legardeur, H.; Masiello-Fonjallaz, G.; Jacot-Guillarmod, M.; Mathevet, P. Safety and Efficacy of an Atraumatic Uterine Cervical Traction Device: A Pilot Study. Front. Med. 2021, 8, 742182. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Netherton, R.; Majumder, K.; Edi-Osagie, E.; Sajjad, Y. Methods Employed to Overcome Difficult Embryo Transfer during Assisted Reproduction Treatment. Arch. Gynecol. Obstet. 2015, 292, 255–262. [Google Scholar] [CrossRef]
- Xu, J.; Yin, M.-N.; Chen, Z.-H.; Yang, L.; Ye, D.-S.; Sun, L. Embryo Retention Significantly Decreases Clinical Pregnancy Rate and Live Birth Rate: A Matched Retrospective Cohort Study. Fertil. Steril. 2020, 114, 787–791. [Google Scholar] [CrossRef]
- Alvarez, M.; Martínez, F.; Bourroul, F.M.; Polyzos, N.P.; Solé, M.; Parriego, M.; Rodríguez, I.; Barri, P.N.; Coroleu, B. Effect of Embryo Transfer Difficulty on Live Birth Rates Studied in Vitrified-Warmed Euploid Blastocyst Transfers. Reprod. Biomed. Online 2019, 39, 940–946. [Google Scholar] [CrossRef]
- Pantou, A.; Simopoulou, M.; Sfakianoudis, K.; Giannelou, P.; Rapani, A.; Maziotis, E.; Grigoriadis, S.; Tsioulou, P.; Syrkos, S.; Souretis, K.; et al. The Role of Laparoscopic Investigation in Enabling Natural Conception and Avoiding in Vitro Fertilization Overuse for Infertile Patients of Unidentified Aetiology and Recurrent Implantation Failure Following in Vitro Fertilization. J. Clin. Med. 2019, 8, 548. [Google Scholar] [CrossRef]
- Pantos, K.; Sfakianoudis, K.; Grigoriadis, S.; Maziotis, E.; Tsioulou, P.; Rapani, A.; Giannelou, P.; Atzampos, A.; Koulouraki, S.; Koutsilieris, M.; et al. Could the Age Difference of a Single Calendar Year between Patients Undergoing IVF at 34, 35 or at 36 Years Old Affect the IVF Outcome? A Retrospective Data Analysis. Medicina 2020, 56, 92. [Google Scholar] [CrossRef] [PubMed]
- Veeck, L.L. (Ed.) An Atlas of Human Gametes and Conceptuses: An Illustrated Reference for Assisted Reproductive Technology; Taylor & Francis: New York, NY, USA, 1999. [Google Scholar]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst Score Affects Implantation and Pregnancy Outcome: Towards a Single Blastocyst Transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef]
- Zhan, Q.; Sierra, E.T.; Malmsten, J.; Ye, Z.; Rosenwaks, Z.; Zaninovic, N. Blastocyst Score, a Blastocyst Quality Ranking Tool, Is a Predictor of Blastocyst Ploidy and Implantation Potential. F&S Rep. 2020, 1, 133–141. [Google Scholar] [CrossRef]
- Gursu, T.; Eraslan, A.; Angun, B.; Celik, H.G.; Yeh, J.; Bastu, E. Comparison of Pregnancy Outcomes of Cervical Mucus Washing with Physiologic Saline Solution or G-Rinse Medium Solution, in Elective Single-Embryo Transfers. J. Assist. Reprod. Genet. 2023, 40, 865–871. [Google Scholar] [CrossRef]
- Larue, L.; Bernard, L.; Moulin, J.; Massari, A.; Cassuto, N.-G.; Bouret, D.; Keromnes, G. Evaluation of a Strategy for Difficult Embryo Transfers from a Prospective Series of 2,046 Transfers. F&S Rep. 2021, 2, 43–49. [Google Scholar] [CrossRef]
- Larue, L.; Keromnes, G.; Massari, A.; Roche, C.; Bouret, D.; Cassuto, N.G.; Ayel, J.P. Anatomical Causes of Difficult Embryo Transfer during in Vitro Fertilization. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Saravelos, S.H.; Wong, A.W.; Kong, G.W.; Huang, J.; Klitzman, R.; Li, T.-C. Pain during Embryo Transfer Is Independently Associated with Clinical Pregnancy in Fresh/Frozen Assisted Reproductive Technology Cycles. J. Obstet. Gynaecol. Res. 2016, 42, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Lesny, P.; Killick, S.R.; Robinson, J.; Raven, G.; Maguiness, S.D. Junctional Zone Contractions and Embryo Transfer: Is It Safe to Use a Tenaculum? Hum. Reprod. 1999, 14, 2367–2370. [Google Scholar] [CrossRef] [PubMed]
- Drakeley, A.J.; Jorgensen, A.; Sklavounos, J.; Aust, T.; Gazvani, R.; Williamson, P.; Kingsland, C.R. A Randomized Controlled Clinical Trial of 2295 Ultrasound-Guided Embryo Transfers. Hum. Reprod. 2008, 23, 1101–1106. [Google Scholar] [CrossRef]
- Strawn, E.Y.; Tucker, A.; Roesler, M.; Granlund, A.; Gunnarson, C.; Eastwood, D. Tenaculum and Local Anesthetic Use for Embryo Transfer (ET) Does Not Compromise Pregnancy Rates in Fresh in Vitro Fertilization (IVF) Cycles and Embryo Thaw and Transfer (ET-T) Cycles. Fertil. Steril. 2002, 78, S144. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female Age-Related Fertility Decline. Committee Opinion No. 589. Fertil. Steril. 2014, 101, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Cimadomo, D.; Fabozzi, G.; Vaiarelli, A.; Ubaldi, N.; Ubaldi, F.M.; Rienzi, L. Impact of Maternal Age on Oocyte and Embryo Competence. Front. Endocrinol. 2018, 9, 327. [Google Scholar] [CrossRef]
- Huang, J.; Tao, Y.; Zhang, J.; Yang, X.; Wu, J.; Kuang, Y.; Wang, Y. Poor Embryo Quality Is Associated With A Higher Risk of Low Birthweight in Vitrified-Warmed Single Embryo Transfer Cycles. Front. Physiol. 2020, 11, 415. [Google Scholar] [CrossRef]
- Kirillova, A.; Lysenkov, S.; Farmakovskaya, M.; Kiseleva, Y.; Martazanova, B.; Mishieva, N.; Abubakirov, A.; Sukhikh, G. Should We Transfer Poor Quality Embryos? Fertil. Res. Pract. 2020, 6, 2. [Google Scholar] [CrossRef]
- Zhu, J.; Lian, Y.; Li, M.; Chen, L.; Liu, P.; Qiao, J. Does IVF Cleavage Stage Embryo Quality Affect Pregnancy Complications and Neonatal Outcomes in Singleton Gestations after Double Embryo Transfers? J. Assist. Reprod. Genet. 2014, 31, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.F.; Wan, F.; Huang, R.; Zhang, H.W. Factors Predicting the Cumulative Outcome of IVF/ICSI Treatment: A Multivariable Analysis of 2450 Patients. Hum. Reprod. 2011, 26, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Sainte-Rose, R.; Petit, C.; Dijols, L.; Frapsauce, C.; Guerif, F. Extended Embryo Culture Is Effective for Patients of an Advanced Maternal Age. Sci. Rep. 2021, 11, 13499. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Chen, C.-D.; Tsai, Y.-Y.; Chang, L.-J.; Ho, H.-N.; Yang, Y.-S. Embryo Quality Is More Important for Younger Women Whereas Age Is More Important for Older Women with Regard to in Vitro Fertilization Outcome and Multiple Pregnancy. Fertil. Steril. 2006, 86, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine and the Practice Committee for the Society for Assisted Reproductive Technologies. Guidance on the Limits to the Number of Embryos to Transfer: A Committee Opinion. Fertil. Steril. 2021, 116, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Racca, A.; Drakopoulos, P.; Van Landuyt, L.; Willem, C.; Santos-Ribeiro, S.; Tournaye, H.; Blockeel, C.; Polyzos, N.P. Single and Double Embryo Transfer Provide Similar Live Birth Rates in Frozen Cycles. Gynecol. Endocrinol. 2020, 36, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.; Mascarenhas, M.; Kirubakaran, R.; Bhattacharya, S. Number of Embryos for Transfer Following in Vitro Fertilisation or Intra-cytoplasmic Sperm Injection. Cochrane Database Syst. Rev. 2020, 8, CD003416. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, X.; Gui, T.; Tao, T.; Xiong, W. Transfer of a Poor-Quality along with a Good-Quality Embryo on in Vitro Fertilization/Intracytoplasmic Sperm Injection-Embryo Transfer Clinical Outcomes: A Systematic Review and Meta-Analysis. Fertil. Steril. 2022, 118, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Peng, Y.; Hu, L.; Wang, X.; Xiong, Y.; Tang, Y.; Tan, J.; Gong, F. Comparisons of Benefits and Risks of Single Embryo Transfer versus Double Embryo Transfer: A Systematic Review and Meta-Analysis. Reprod. Biol. Endocrinol. 2022, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, H.; Sheng, X.; Liang, D.; Tan, H.; Xia, J. Pregnancy-Related Complications and Adverse Pregnancy Outcomes in Multiple Pregnancies Resulting from Assisted Reproductive Technology: A Meta-Analysis of Cohort Studies. Fertil. Steril. 2015, 103, 1492–1508.e7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, L.; Yang, T.; Yu, H.; Wang, H.; Qin, J. Multiple Pregnancies Achieved with IVF/ICSI and Risk of Specific Congenital Malformations: A Meta-Analysis of Cohort Studies. Reprod. Biomed. Online 2018, 36, 472–482. [Google Scholar] [CrossRef]
- Bourdon, M.; Pocate-Cheriet, K.; Finet de Bantel, A.; Grzegorczyk-Martin, V.; Amar Hoffet, A.; Arbo, E.; Poulain, M.; Santulli, P. Day 5 versus Day 6 Blastocyst Transfers: A Systematic Review and Meta-Analysis of Clinical Outcomes. Hum. Reprod. 2019, 34, 1948–1964. [Google Scholar] [CrossRef]
- Simopoulou, M.; Sfakianoudis, K.; Tsioulou, P.; Rapani, A.; Maziotis, E.; Giannelou, P.; Grigoriadis, S.; Pantou, A.; Nikolettos, K.; Vlahos, N.; et al. Should the Flexibility Enabled by Performing a Day-4 Embryo Transfer Remain as a Valid Option in the IVF Laboratory? A Systematic Review and Network Meta-Analysis. J. Assist. Reprod. Genet. 2019, 36, 1049–1061. [Google Scholar] [CrossRef]
- Murphy, A.; Lapczynski, M.; Proctor, G.; Meyer, E.C.; Glynn, T.; Domar, A.; Gameiro, S.; Palmer, G.; Collins, M.G. P-572 The Occupational Challenges Reported by UK Embryologists: Stress, Fatigue, and Burnout. Human. Reprod. 2023, 38, dead093.906. [Google Scholar] [CrossRef]


| ET Parameters | Mean |
|---|---|
| Age | 35.5 (±3.51) |
| Mean number of embryos transferred | 2.15 (±0.58) |
| Single ETs | 10.45% (148/1417) |
| Double ETs | 63.37% (898/1417) |
| Triple ETs | 26.18% (371/1417) |
| Quality of embryos | |
| GOOD | 22.23% (315/1417) |
| FAIR | 59.50% (843/1417) |
| POOR | 18.27% (259/1417) |
| Day of transfer | |
| DAY 6 | 6.07% (86/1417) |
| DAY 5 | 53.42% (757/1417) |
| DAY 4 | 17.3% (245/1417) |
| DAY 3 | 21.59% (306/1417) |
| DAY 2 | 1.62% (23/1417) |
| Ultrasound employment | 82.99% (1176/1417) |
| Resistance | |
| NO | 87.86% (1245/1417) |
| MILD | 9.39% (133/1417) |
| SIGNIFICANT | 2.75% (39/1417) |
| Discomfort | |
| NO | 94.07% (1333/1417) |
| MILD | 5.01% (71/1417) |
| SIGNIFICANT | 0.92% (13/1417) |
| Use of Tenaculum | 3.95% (56/1415) |
| Bleeding | |
| NO | 83.95% (1189/1417) |
| MILD | 15% (213/1417) |
| SIGNIFICANT | 1.05% (15/1417) |
| Presence of Mucus | 4.52% (64/1417) |
| Catheter Reload | 0.56% (8/1417) |
| Positive β-hCG | 48.27% (684/1417) |
| Single ETs | 37.16% (55/148) |
| Double ETs | 50.89% (457/898) |
| Triple ETs | 46.36% (172/371) |
| Clinical pregnancy | 38.11% (540/1417) |
| Single ETs | 26.35% (39/148) |
| Double ETs | 40.65% (365/898) |
| Triple ETs | 36.66% (136/371) |
| Live birth | 33.73% (478/1417) |
| Single ETs | 26.35% (39/148) |
| Double ETs | 35.97% (323/898) |
| Triple ETs | 31.27% (116/371) |
| Twins | 10.47% (51/478) |
| Single ETs | 0% (0/39) |
| Double ETs | 11.77% (38/323) |
| Triple ETs | 11.21% (13/116) |
| Estimate | Std. Error | z Value | p-Value | |
|---|---|---|---|---|
| Maternal Age | −0.5832 | 0.2356 | −2.721 | 0.0045 |
| Day of ET | 0.5986 | 0.2786 | 1.918 | 0.0613 |
| Physician | −0.6894 | 1.1050 | −0.624 | 0.5327 |
| Embryologist | 0.7056 | 0.4598 | 1.535 | 0.1249 |
| Mild Bleeding | −0.1498 | 0.2355 | −0.636 | 0.5747 |
| Significant Bleeding | −1.8464 | 1.4317 | −1.290 | 0.1972 |
| Mild Resistance | 0.2771 | 0.3272 | 0.847 | 0.3970 |
| Significant Resistance | −0.3573 | 0.8058 | −0.443 | 0.6574 |
| Catheter Reload | 1.2020 | 0.8697 | 1.382 | 0.1667 |
| Employment of Tenaculum | 0.4693 | 0.4177 | 1.124 | 0.2611 |
| Mild Discomfort | −1.0431 | 1.1427 | −0.913 | 0.3613 |
| Significant Discomfort | −1.6868 | 0.9283 | −1.817 | 0.0692 |
| Presence of Mucus | 0.2094 | 0.4696 | 0.446 | 0.6557 |
| Poor-Quality Embryo | −0.6484 | 0.2377 | −2.928 | 0.0006 |
| Good-Quality Embryo | −0.5918 | 0.2366 | −2.803 | 0.0031 |
| Number of Embryos | −0.1512 | 0.1141 | −1.325 | 0.1850 |
| Summary Square | Mean Square | F Value | p-Value | |
|---|---|---|---|---|
| Day of ET | 0.673 | 0.67272 | 4.4379 | 0.035340 |
| Maternal Age | 1.612 | 1.61206 | 10.6349 | 0.001139 |
| Embryologist | 4.256 | 0.15764 | 1.04 | 0.408585 |
| Physician | 5.866 | 0.21725 | 1.4332 | 0.070346 |
| Bleeding | 0.108 | 0.05393 | 0.3558 | 0.700684 |
| Resistance | 0.070 | 0.03478 | 0.2295 | 0.794987 |
| Reload | 0.417 | 0.41715 | 2.7519 | 0.097378 |
| Tenaculum | 0.175 | 0.17467 | 1.1523 | 0.283264 |
| Ease | 0.097 | 0.04831 | 0.3187 | 0.727146 |
| Discomfort | 0.393 | 0.19641 | 1.2958 | 0.274045 |
| Mucus | 0.074 | 0.07351 | 0.4849 | 0.486317 |
| Ultrasound | 0.222 | 0.22155 | 1.4616 | 0.226894 |
| Embryo Quality | 2.017 | 0.67222 | 4.4347 | 0.004142 |
| Number of Embryos | 1.303 | 1.30321 | 3.9989 | 0.015765 |
| Estimate | Std. Error | z Value | p-Value | |
|---|---|---|---|---|
| Maternal Age | 0.07019 | 0.04242 | 1.655 | 0.098 |
| Day of ET | 0.08127 | 0.10687 | 0.76 | 0.447 |
| Physician | −0.6894 | 1.1050 | −0.624 | 0.5327 |
| Embryologist | 0.7056 | 0.4598 | 1.535 | 0.1249 |
| Mild Bleeding | −0.06083 | 0.26036 | −0.234 | 0.815 |
| Significant Bleeding | −16.0603 | 1982.885 | −0.008 | 0.994 |
| Mild Resistance | 0.35054 | 0.38269 | 0.916 | 0.36 |
| Significant Resistance | −0.14422 | 0.7284 | −0.198 | 0.843 |
| Catheter Reload | 0.05018 | 1.05554 | 0.048 | 0.962 |
| Employment of Tenaculum | 0.45905 | 0.53199 | 0.863 | 0.388 |
| Mild Discomfort | −0.19509 | 0.49121 | −0.397 | 0.691 |
| Significant Discomfort | −16.6224 | 2426.842 | −0.007 | 0.995 |
| Presence of Mucus | 0.2094 | 0.4696 | 0.446 | 0.6557 |
| Top-Quality Embryo | 0.25237 | 0.22359 | 1.947 | 0.0584 |
| Number of Embryos | 0.17532 | 0.18942 | 0.926 | 0.355 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfakianoudis, K.; Maziotis, E.; Trypidi, A.; Grigoriadis, S.; Vaxevanoglou, T.; Angeli, I.; Rapani, A.; Kotsifaki, A.; Pistola, K.; Pantou, A.; et al. Embryo Transfer Procedural Parameters Do Not Predict IVF Cycle Outcome. J. Clin. Med. 2024, 13, 1312. https://doi.org/10.3390/jcm13051312
Sfakianoudis K, Maziotis E, Trypidi A, Grigoriadis S, Vaxevanoglou T, Angeli I, Rapani A, Kotsifaki A, Pistola K, Pantou A, et al. Embryo Transfer Procedural Parameters Do Not Predict IVF Cycle Outcome. Journal of Clinical Medicine. 2024; 13(5):1312. https://doi.org/10.3390/jcm13051312
Chicago/Turabian StyleSfakianoudis, Konstantinos, Evangelos Maziotis, Anna Trypidi, Sokratis Grigoriadis, Terpsithea Vaxevanoglou, Irene Angeli, Anna Rapani, Amalia Kotsifaki, Kalliopi Pistola, Agni Pantou, and et al. 2024. "Embryo Transfer Procedural Parameters Do Not Predict IVF Cycle Outcome" Journal of Clinical Medicine 13, no. 5: 1312. https://doi.org/10.3390/jcm13051312