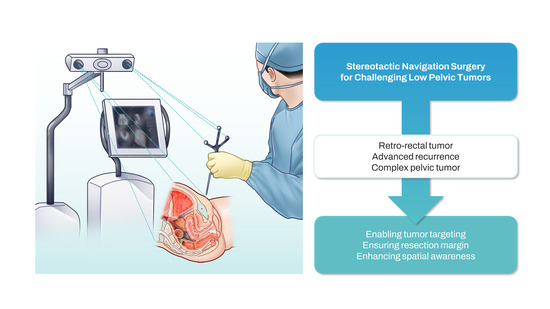
Stereotactic Navigation-Assisted Laparoscopic Resection of Challenging Low Pelvic Tumors: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Rationale
2.3. Image Acquisition
2.4. Operating Theatre Setup
3. Results
3.1. Case 1: Retro-Rectal Tumor
3.2. Case 2: Rectal Cancer Local Recurrence
3.3. Case 3: Advanced Anal Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orringer, D.A.; Golby, A.; Jolesz, F. Neuronavigation in the surgical management of brain tumors: Current and future trends. Expert Rev. Med. Devices 2012, 9, 491–500. [Google Scholar] [CrossRef] [PubMed]
- van der List, J.P.; Chawla, H.; Joskowicz, L.; Pearle, A.D. Current state of computer navigation and robotics in unicompartmental and total knee arthroplasty: A systematic review with meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3482–3495. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Romagnolo, L.; Wijsmuller, A.; Gonzalez, C.; Agnus, V.; Lucchesi, F.R.; Melani, A.; Marescaux, J.; Dallemagne, B. Stereotactic Pelvic Navigation With Augmented Reality for Transanal Total Mesorectal Excision. Dis. Colon Rectum 2019, 62, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Nassif, G.; Larach, S. Stereotactic navigation for TAMIS-TME: Opening the gateway to frameless, image-guided abdominal and pelvic surgery. Surg. Endosc. 2015, 29, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Parra-Davila, E.; Melani, A.G.F.; Romagnolo, L.G.; Larach, S.W.; Marescaux, J. Robotic-assisted stereotactic real-time navigation: Initial clinical experience and feasibility for rectal cancer surgery. Tech. Coloproctol. 2019, 23, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Martin-Perez, B.; Larach, S. Image-guided real-time navigation for transanal total mesorectal excision: A pilot study. Tech. Coloproctol. 2015, 19, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Tilahun, Y.; Monson, J.R. Real-time stereotactic navigation for the laparoscopic excision of a pelvic neoplasm. Tech. Coloproctol. 2016, 20, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Nijkamp, J.; Kuhlmann, K.F.D.; Ivashchenko, O.; Pouw, B.; Hoetjes, N.; Lindenberg, M.A.; Aalbers, A.G.J.; Beets, G.L.; van Coevorden, F.; Ko, K.N.; et al. Prospective study on image-guided navigation surgery for pelvic malignancies. J. Surg. Oncol. 2019, 119, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, K.; Kobayashi, E.; Tsukihara, H.; Nozawa, H.; Kawai, K.; Sasaki, K.; Murono, K.; Ishihara, S.; Sakuma, I. Stereotactic Navigation System for Laparoscopic Lateral Pelvic Lymph Node Dissection. Dis. Colon Rectum 2021, 64, e372–e377. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Akasu, T.; Mizusawa, J.; Saito, N.; Kinugasa, Y.; Kanemitsu, Y.; Ohue, M.; Fujii, S.; Shiozawa, M.; Yamaguchi, T.; et al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): Results from a multicentre, randomised controlled, noninferiority trial. Lancet Oncol. 2012, 13, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, K.; Kobayashi, E.; Sasaki, K.; Nozawa, H.; Kawai, K.; Murono, K.; Sakuma, I.; Ishihara, S. Stereotactic navigation using registration based on intra-abdominal landmarks in robotic-assisted lateral pelvic lymph node dissection. Tech. Coloproctol. 2022, 26, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Hojo, D.; Emoto, S.; Kawai, K.; Nozawa, H.; Hata, K.; Tanaka, T.; Ishihara, S. Potential Usefulness of Three-dimensional Navigation Tools for the Resection of Intra-abdominal Recurrence of Colorectal Cancer. J. Gastrointest. Surg. 2020, 24, 1682–1685. [Google Scholar] [CrossRef] [PubMed]
- Solbakken, A.M.; Sellevold, S.; Spasojevic, M.; Julsrud, L.; Emblemsvåg, H.L.; Reims, H.M.; Sørensen, O.; Thorgersen, E.B.; Fauske, L.; Ågren, J.S.M.; et al. Navigation-Assisted Surgery for Locally Advanced Primary and Recurrent Rectal Cancer. Ann. Surg. Oncol. 2023, 30, 7602–7611. [Google Scholar] [CrossRef] [PubMed]
- Kok, E.N.D.; van Veen, R.; Groen, H.C.; Heerink, W.J.; Hoetjes, N.J.; van Werkhoven, E.; Beets, G.L.; Aalbers, A.G.J.; Kuhlmann, K.F.D.; Nijkamp, J.; et al. Association of image-guided navigation with complete resection rate in patients with locally advanced primary and recurrent rectal cancer: A nonrandomized controlled trial. JAMA Netw. Open 2020, 3, e208522. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Larach, S.W.; Monson, J.R. Stereotactic navigation for TAMIS-TME. Minim. Invasive Ther. Allied Technol. 2016, 25, 271–277. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piozzi, G.N.; Kwak, J.-M.; Kim, J.-S.; Baek, S.-J.; Kim, J.; Kim, S.-H. Stereotactic Navigation-Assisted Laparoscopic Resection of Challenging Low Pelvic Tumors: A Case Series. J. Clin. Med. 2024, 13, 1233. https://doi.org/10.3390/jcm13051233
Piozzi GN, Kwak J-M, Kim J-S, Baek S-J, Kim J, Kim S-H. Stereotactic Navigation-Assisted Laparoscopic Resection of Challenging Low Pelvic Tumors: A Case Series. Journal of Clinical Medicine. 2024; 13(5):1233. https://doi.org/10.3390/jcm13051233
Chicago/Turabian StylePiozzi, Guglielmo Niccolò, Jung-Myun Kwak, Ji-Seon Kim, Se-Jin Baek, Jin Kim, and Seon-Hahn Kim. 2024. "Stereotactic Navigation-Assisted Laparoscopic Resection of Challenging Low Pelvic Tumors: A Case Series" Journal of Clinical Medicine 13, no. 5: 1233. https://doi.org/10.3390/jcm13051233