The Relationship between Sleep Parameters Measured by Polysomnography and Selected Neurotrophic Factors
Abstract
:1. Introduction
2. Materials and Methods
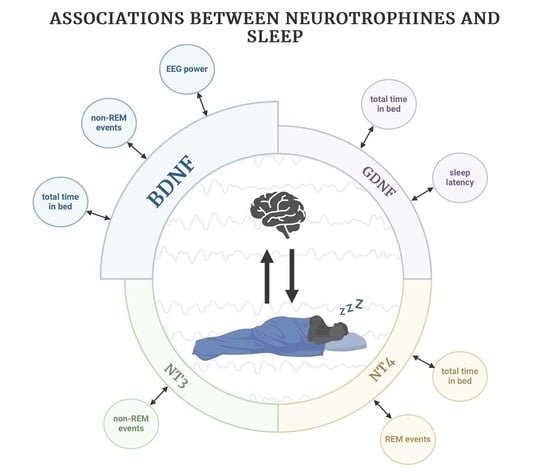
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Üstün, T.B.; Privett, M.; Lecrubier, Y.; Weiller, E.; Simon, G.; Korten, A.; Bassett, S.S.; Maier, W.; Sartorius, N. Form, frequency and burden of sleep problems in general health care: A report from the WHO Collaborative Study on Psychological Problems in General Health Care. Eur. Psychiatry 1996, 11, 5s–10s. [Google Scholar] [CrossRef]
- Jansson-Frojmark, M.; Lindblom, K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J. Psychosom. Res. 2008, 64, 443–449. [Google Scholar] [CrossRef]
- Mueller, A.D.; Meerlo, P.; McGinty, D.; Mistlberger, R.E. Sleep and adult neurogenesis: Implications for cognition and mood. Curr. Top Behav. Neurosci. 2015, 25, 151–181. [Google Scholar] [CrossRef]
- Neylan, T.C.; Mueller, S.G.; Wang, Z.; Metzler, T.J.; Lenoci, M.; Truran, D.; Marmar, C.R.; Weiner, M.W.; Schuff, N. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol. Psychiatry 2010, 68, 494–496. [Google Scholar] [CrossRef]
- Chang, H.M.; Wu, H.C.; Sun, Z.G.; Lian, F.; Leung, P.C.K. Neurotrophins and glial cell line-derived neurotrophic factor in the ovary: Physiological and pathophysiological implications. Hum. Reprod. Update 2019, 25, 224–242. [Google Scholar] [CrossRef]
- Castren, E.; Antila, H. Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef]
- Bartkowska, K.; Turlejski, K.; Djavadian, R.L. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol. Exp. 2010, 70, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Holsboer-Trachsler, E.; Eckert, A. BDNF in sleep, insomnia, and sleep deprivation. Ann. Med. 2016, 48, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Kanai, M.; Kijima, K.; Akaba, K.; Hashimoto, M.; Hasegawa, H.; Otaki, S.; Koizumi, T.; Kusuda, S.; Ogawa, Y.; et al. Molecular analysis of congenital central hypoventilation syndrome. Hum. Genet. 2003, 114, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Kushikata, T.; Kubota, T.; Fang, J.; Krueger, J.M. Glial cell line-derived neurotrophic factor promotes sleep in rats and rabbits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1001–R1006. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.T.; Chen, W.H.; Shi, L.; Lin, X.; Tabarak, S.; Chen, S.J.; Que, J.Y.; Bao, Y.P.; Tang, X.D.; Shi, J.; et al. Objective sleep duration is associated with cognitive deficits in primary insomnia: BDNF may play a role. Sleep 2019, 42, zsy192. [Google Scholar] [CrossRef] [PubMed]
- Gorgulu, Y.; Caliyurt, O.; Kose Cinar, R.; Sonmez, M.B. Acute sleep deprivation immediately increases serum GDNF, BDNF and VEGF levels in healthy subjects. Sleep Biol. Rhythm. 2022, 20, 73–79. [Google Scholar] [CrossRef]
- Wang, W.H.; He, G.P.; Xiao, X.P.; Gu, C.; Chen, H.Y. Relationship between brain-derived neurotrophic factor and cognitive function of obstructive sleep apnea/hypopnea syndrome patients. Asian Pac. J. Trop. Med. 2012, 5, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Zhang, Q.L.; Huang, C.L.; Wu, W.Q.; Sun, J.W. Association of Decreased Serum BDNF With Restless Legs Syndrome in Parkinson’s Disease Patients. Front. Neurol. 2021, 12, 734570. [Google Scholar] [CrossRef]
- Klein, A.B.; Jennum, P.; Knudsen, S.; Gammeltoft, S.; Mikkelsen, J.D. Increased serum brain-derived neurotrophic factor (BDNF) levels in patients with narcolepsy. Neurosci. Lett. 2013, 544, 31–35. [Google Scholar] [CrossRef]
- Biasiucci, A.; Franceschiello, B.; Murray, M.M. Electroencephalography. Curr. Biol. 2019, 29, R80–R85. [Google Scholar] [CrossRef]
- Lambert, I.; Peter-Derex, L. Spotlight on Sleep Stage Classification Based on EEG. Nat. Sci. Sleep 2023, 15, 479–490. [Google Scholar] [CrossRef]
- Chikhi, S.; Matton, N.; Blanchet, S. EEG power spectral measures of cognitive workload: A meta-analysis. Psychophysiology 2022, 59, e14009. [Google Scholar] [CrossRef] [PubMed]
- Faraguna, U.; Vyazovskiy, V.V.; Nelson, A.B.; Tononi, G.; Cirelli, C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J. Neurosci. 2008, 28, 4088–4095. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Cho, S.E.; Moon, J.Y.; Kim, S.I.; Kim, J.W.; Kang, S.G. Difference in spectral power density of sleep electroencephalography between individuals without insomnia and frequent hypnotic users with insomnia complaints. Sci. Rep. 2022, 12, 2117. [Google Scholar] [CrossRef]
- Gabryelska, A.; Feige, B.; Riemann, D.; Spiegelhalder, K.; Johann, A.; Bialasiewicz, P.; Hertenstein, E. Can spectral power predict subjective sleep quality in healthy individuals? J. Sleep Res. 2019, 28, e12848. [Google Scholar] [CrossRef]
- Roy, N.; Barry, R.J.; Fernandez, F.E.; Lim, C.K.; Al-Dabbas, M.A.; Karamacoska, D.; Broyd, S.J.; Solowij, N.; Chiu, C.L.; Steiner, G.Z. Electrophysiological correlates of the brain-derived neurotrophic factor (BDNF) Val66Met polymorphism. Sci. Rep. 2020, 10, 17915. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Wysokiński, A. NeuroAnalyzer. Available online: https://neuroanalyzer.org/ (accessed on 8 January 2024).
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Nayak, C.S.; Anilkumar, A.C. EEG Normal Sleep. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Giese, M.; Unternährer, E.; Hüttig, H.; Beck, J.; Brand, S.; Calabrese, P.; Holsboer-Trachsler, E.; Eckert, A. BDNF: An indicator of insomnia? Mol. Psychiatry 2014, 19, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.K.; Ilchibaeva, T.V.; Naumenko, V.S. Neurotrophic Factors (BDNF and GDNF) and the Serotonergic System of the Brain. Biochemistry 2017, 82, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Proenca, C.C.; Song, M.; Lee, F.S. Differential effects of BDNF and neurotrophin 4 (NT4) on endocytic sorting of TrkB receptors. J. Neurochem. 2016, 138, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Schloesser, R.J.; Jimenez, D.V.; Weinberger, D.R.; Lu, B. Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol. Brain 2011, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.M.; Chambers, J.; Barnes, A.K.; Datta, S. Changes in Brain-Derived Neurotrophic Factor Expression Influence Sleep-Wake Activity and Homeostatic Regulation of Rapid Eye Movement Sleep. Sleep 2018, 41, zsx194. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.K.; Koul-Tiwari, R.; Garner, J.M.; Geist, P.A.; Datta, S. Activation of brain-derived neurotrophic factor-tropomyosin receptor kinase B signaling in the pedunculopontine tegmental nucleus: A novel mechanism for the homeostatic regulation of rapid eye movement sleep. J. Neurochem. 2017, 141, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Korai, S.A.; Ranieri, F.; Di Lazzaro, V.; Papa, M.; Cirillo, G. Neurobiological After-Effects of Low Intensity Transcranial Electric Stimulation of the Human Nervous System: From Basic Mechanisms to Metaplasticity. Front. Neurol. 2021, 12, 587771. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Wysokiński, A.; Szczepocka, E.; Szczakowska, A. Improved cognitive performance, increased theta, alpha, beta and decreased delta powers after cognitive rehabilitation augmented with tDCS in a patient with post-COVID-19 cognitive impairment (brain-fog). Psychiatry Res. Case Rep. 2023, 2, 100164. [Google Scholar] [CrossRef]
- Wysokiński, A. Successful replacement of electroconvulsive treatment (ECT) with transcranial direct current stimulation (tDCS) in severe, treatment-refractory catatonic schizophrenia: Case study. Schizophr. Res. 2020, 220, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Keeser, D.; Padberg, F.; Reisinger, E.; Pogarell, O.; Kirsch, V.; Palm, U.; Karch, S.; Möller, H.J.; Nitsche, M.A.; Mulert, C. Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: A standardized low resolution tomography (sLORETA) study. Neuroimage 2011, 55, 644–657. [Google Scholar] [CrossRef]
- Nanobashvili, A.; Melin, E.; Emerich, D.; Tornøe, J.; Simonato, M.; Wahlberg, L.; Kokaia, M. Unilateral ex vivo gene therapy by GDNF in epileptic rats. Gene Ther. 2019, 26, 65–74. [Google Scholar] [CrossRef]
- Kushikata, T.; Kubota, T.; Fang, J.; Krueger, J.M. Neurotrophins 3 and 4 enhance non-rapid eye movement sleep in rabbits. Neurosci. Lett. 2003, 346, 161–164. [Google Scholar] [CrossRef]
| Women (n, %) Men (n, %) | 41 (50.62) 40 (49.38) |
| Age | 24.00 (22.00–26.00) |
| BMI | 22.77 (21.47–24.76) |
| Smoking (n, %) | 9 (11.11) |
| Higher education (n, %) | 39 (48.15) |
| TIB (min) | 536.00 (515.00–558.80) |
| TST (min) | 407.00 (360.00–470.50) |
| REM duration (min) | 91.00 (67.50–121.50) |
| NREM duration (min) | 332.50 (285.50–352.50) |
| Sleep latency (min) | 35.00 (22.00–59.00) |
| Latencies REM (min) | 142.00 (94.50–185.50) |
| Sleep efficiency (%) | 78.60 (70.20–86.20) |
| NREM events | 3.00 (1.00–7.00) |
| REM events | 3.00 (1.00–6.00) |
| TIB | TST | NREM Duration | REM Duration | Sleep Latency | REM Latencies | Sleep Efficiency | Non-REM Events | REM Events | |
|---|---|---|---|---|---|---|---|---|---|
| BDNF | −0.37; 0.001 | −0.21; 0.066 | −0.15; 0.203 | −0.19; 0.096 | 0.17; 0.131 | 0.06; 0.593 | −0.02; 0.837 | −0.24; 0.037 | −0.15; 0.206 |
| mRNA BDNF | 0.24; 0.041 | 0.04; 0.709 | 0.05; 0.675 | 0.01; 0.945 | −0.14; 0.225 | −0.13; 0.273 | −0.05; 0.684 | −0.05; 0.673 | −0.18; 0.141 |
| NT3 | 0.05; 0.689 | −0.2; 0.085 | −0.17; 0.146 | −0.16; 0.157 | 0.01; 0.914 | 0.17; 0.149 | −0.01; 0.91 | −0.24; 0.041 | −0.09; 0.449 |
| mRNA NT3 | 0.11; 0.357 | 0.1; 0.416 | 0.13; 0.286 | −0.04; 0.748 | −0.2; 0.091 | 0; 0.985 | 0.05; 0.679 | −0.19; 0.11 | −0.22; 0.062 |
| NT4 | −0.32; 0.005 | −0.05; 0.659 | −0.15; 0.191 | 0.11; 0.325 | 0.04; 0.762 | −0.13; 0.247 | −0.1; 0.392 | 0.16; 0.162 | 0.32; 0.005 |
| mRNA NT4 | 0.01; 0.901 | 0.07; 0.563 | 0.09; 0.469 | −0.04; 0.745 | −0.07; 0.556 | −0.07; 0.569 | 0.05; 0.695 | −0.03; 0.776 | −0.2; 0.095 |
| GDNF | −0.25; 0.032 | −0.17; 0.141 | −0.18; 0.119 | 0.01; 0.943 | −0.08; 0.517 | −0.2; 0.091 | −0.07; 0.572 | −0.1; 0.369 | −0.18; 0.13 |
| mRNA GDNF | 0.09; 0.431 | 0.04; 0.719 | 0.07; 0.587 | −0.05; 0.675 | −0.25; 0.035 | 0.07; 0.539 | 0; 0.978 | −0.15; 0.204 | −0.17; 0.162 |
| n | BDNF | mRNA BDNF | NT3 | mRNA NT3 | NT4 | mRNA NT4 | GDNF | mRNA GDNF | |
|---|---|---|---|---|---|---|---|---|---|
| REM delta F4A1 | 43 | −0.48; 0.001 | 0.09; 0.590 | 0.05; 0.74 | 0.02; 0.896 | −0.19; 0.238 | 0.07; 0.69 | 0.09; 0.567 | −0.04; 0.813 |
| REM delta F3A2 | 53 | −0.37; 0.008 | 0.15; 0.312 | 0.07; 0.637 | 0.03; 0.855 | −0.11; 0.43 | 0.14; 0.338 | 0.18; 0.205 | −0.02; 0.867 |
| REM delta C4A1 | 43 | −0.43; 0.005 | 0.14; 0.392 | 0.04; 0.781 | 0.03; 0.85 | −0.11; 0.476 | 0; 0.978 | 0.11; 0.494 | 0.02; 0.917 |
| REM delta C3A2 | 53 | −0.36; 0.01 | 0.15; 0.317 | 0.06; 0.666 | 0.03; 0.822 | −0.11; 0.424 | 0.14; 0.342 | 0.19; 0.184 | −0.02; 0.912 |
| REM delta O2A1 | 40 | −0.38; 0.017 | 0.09; 0.582 | −0.02; 0.923 | 0.03; 0.848 | −0.11; 0.506 | 0.05; 0.785 | 0.17; 0.298 | 0; 0.991 |
| REM delta O1A2 | 46 | −0.36; 0.014 | 0.12; 0.450 | 0.21; 0.169 | −0.04; 0.785 | 0.01; 0.923 | −0.12; 0.436 | 0.14; 0.363 | −0.04; 0.779 |
| REM theta F4A1 | 41 | −0.01; 0.943 | 0.07; 0.683 | −0.08; 0.607 | 0.14; 0.392 | 0.19; 0.236 | −0.03; 0.843 | 0.1; 0.521 | 0.18; 0.277 |
| REM theta F3A2 | 49 | 0.09; 0.543 | −0.03; 0.823 | −0.03; 0.829 | −0.15; 0.323 | 0.11; 0.44 | −0.09; 0.535 | 0.17; 0.239 | −0.04; 0.781 |
| REM theta C4A1 | 39 | 0.04; 0.791 | 0.05; 0.790 | −0.08; 0.633 | 0.16; 0.366 | 0.25; 0.135 | −0.05; 0.778 | 0.08; 0.625 | 0.19; 0.272 |
| REM theta C3A2 | 49 | 0.04; 0.773 | −0.03; 0.867 | −0.08; 0.602 | −0.11; 0.488 | 0.09; 0.563 | −0.08; 0.609 | 0.15; 0.325 | −0.04; 0.807 |
| REM theta O2A1 | 37 | −0.14; 0.414 | 0.21; 0.226 | 0.1; 0.55 | 0.29; 0.1 | −0.04; 0.8 | 0.12; 0.495 | −0.01; 0.944 | 0.26; 0.138 |
| REM theta O1A2 | 39 | −0.28; 0.089 | 0.02; 0.886 | 0.15; 0.384 | −0.04; 0.805 | −0.11; 0.498 | −0.1; 0.553 | 0.09; 0.59 | −0.01; 0.97 |
| REM alpha F4A1 | 39 | −0.11; 0.492 | 0.29; 0.081 | −0.1; 0.544 | 0.12; 0.49 | 0.01; 0.967 | −0.01; 0.972 | 0.01; 0.955 | 0.19; 0.25 |
| REM alpha F3A2 | 50 | 0; 0.987 | 0.03; 0.853 | −0.01; 0.958 | −0.12; 0.425 | −0.01; 0.954 | −0.04; 0.797 | 0.21; 0.143 | −0.08; 0.577 |
| REM alpha C4A1 | 41 | −0.06; 0.715 | 0.2; 0.240 | −0.18; 0.258 | 0.09; 0.577 | 0.06; 0.696 | −0.07; 0.656 | 0.11; 0.498 | 0.14; 0.399 |
| REM alpha C3A2 | 38 | −0.02; 0.897 | 0.04; 0.788 | −0.07; 0.643 | −0.16; 0.284 | −0.02; 0.892 | −0.11; 0.483 | 0.18; 0.221 | −0.12; 0.452 |
| REM alpha O2A1 | 39 | −0.03; 0.855 | 0.14; 0.425 | −0.02; 0.91 | 0.23; 0.182 | 0.09; 0.608 | 0.11; 0.545 | 0.1; 0.568 | 0.2; 0.251 |
| REM alpha O1A2 | 49 | −0.09; 0.58 | −0.07; 0.661 | 0.12; 0.463 | −0.09; 0.61 | 0.01; 0.961 | −0.1; 0.54 | 0.18; 0.27 | −0.09; 0.586 |
| REM beta F4A1 | 44 | −0.22; 0.152 | 0.11; 0.487 | −0.01; 0.967 | 0.07; 0.682 | −0.1; 0.538 | 0.1; 0.527 | 0.27; 0.085 | 0.13; 0.41 |
| REM beta F3A2 | 51 | −0.09; 0.516 | −0.14; 0.333 | 0.07; 0.65 | −0.16; 0.275 | 0.01; 0.949 | 0.01; 0.96 | 0.24; 0.097 | −0.14; 0.34 |
| REM beta C3A2 | 44 | −0.08; 0.568 | 0.11; 0.505 | 0.02; 0.914 | 0.08; 0.629 | 0.02; 0.875 | 0.08; 0.615 | 0.24; 0.087 | 0.17; 0.284 |
| REM beta C4A1 | 51 | −0.18; 0.235 | −0.1; 0.488 | −0.05; 0.739 | −0.1; 0.517 | −0.09; 0.567 | 0.09; 0.546 | 0.3; 0.051 | −0.08; 0.581 |
| REM beta O2A1 | 40 | −0.19; 0.255 | 0.03; 0.868 | −0.04; 0.825 | 0; 0.986 | −0.07; 0.668 | 0.02; 0.906 | 0.25; 0.118 | 0; 0.98 |
| REM beta O1A2 | 44 | −0.28; 0.065 | −0.02; 0.895 | 0.07; 0.661 | 0.07; 0.675 | −0.13; 0.407 | −0.01; 0.972 | 0.2; 0.194 | 0.09; 0.591 |
| NREM delta F4A1 | 44 | −0.23; 0.134 | 0.19; 0.226 | −0.13; 0.401 | 0.13; 0.419 | −0.04; 0.806 | 0.17; 0.285 | 0.13; 0.4 | 0.03; 0.863 |
| NREM delta F3A2 | 52 | −0.42; 0.002 | 0.22; 0.127 | −0.18; 0.196 | 0; 0.989 | −0.2; 0.164 | 0.11; 0.452 | 0.04; 0.769 | −0.12; 0.433 |
| NREM delta C3A2 | 52 | −0.42; 0.002 | 0.22; 0.124 | −0.18; 0.214 | 0; 0.98 | −0.2; 0.158 | 0.11; 0.444 | 0.06; 0.698 | −0.11; 0.44 |
| NREM delta C4A1 | 44 | −0.18; 0.236 | 0.26; 0.099 | −0.11; 0.491 | 0.15; 0.341 | 0.02; 0.897 | 0.15; 0.35 | 0.18; 0.255 | 0.09; 0.563 |
| NREM delta O2A1 | 40 | 0.08; 0.64 | −0.13; 0.431 | −0.01; 0.934 | −0.06; 0.742 | −0.08; 0.62 | −0.18; 0.273 | 0.19; 0.241 | 0.02; 0.886 |
| NREM delta O1A2 | 46 | −0.26; 0.09 | 0.10; 0.532 | 0.16; 0.299 | −0.03; 0.83 | −0.12; 0.444 | −0.18; 0.263 | 0; 0.995 | −0.03; 0.829 |
| NREM theta F4A1 | 44 | 0.1; 0.533 | 0.12; 0.460 | −0.11; 0.474 | 0.05; 0.755 | 0.18; 0.25 | −0.07; 0.663 | 0.05; 0.73 | 0.09; 0.563 |
| NREM theta F3A2 | 52 | −0.1; 0.489 | −0.02; 0.867 | −0.04; 0.755 | −0.09; 0.552 | −0.08; 0.583 | −0.03; 0.826 | 0.09; 0.543 | −0.11; 0.459 |
| NREM theta C4A1 | 44 | 0.06; 0.683 | 0.10; 0.531 | −0.16; 0.308 | 0.04; 0.781 | 0.21; 0.179 | −0.05; 0.737 | 0.05; 0.733 | 0.07; 0.679 |
| NREM theta C3A2 | 52 | −0.15; 0.306 | −0.01; 0.930 | −0.09; 0.547 | −0.07; 0.627 | −0.07; 0.616 | 0.04; 0.763 | 0.12; 0.389 | −0.1; 0.483 |
| NREM theta O2A1 | 40 | 0.25; 0.132 | −0.28; 0.091 | 0.05; 0.754 | −0.15; 0.385 | 0.07; 0.669 | −0.25; 0.136 | 0.18; 0.271 | −0.11; 0.536 |
| NREM theta O1A2 | 45 | −0.06; 0.676 | −0.01; 0.925 | −0.07; 0.655 | −0.01; 0.947 | −0.05; 0.735 | −0.12; 0.44 | −0.09; 0.546 | 0; 0.986 |
| NREM alpha F4A1 | 44 | 0.17; 0.283 | 0.14; 0.390 | −0.12; 0.456 | 0.11; 0.478 | 0.12; 0.444 | −0.06; 0.732 | 0.04; 0.817 | 0.16; 0.308 |
| NREM alpha F3A2 | 51 | 0.1; 0.488 | 0.10; 0.515 | −0.04; 0.758 | 0.01; 0.942 | 0.1; 0.503 | 0; 0.982 | 0.01; 0.942 | 0; 0.999 |
| NREM alpha C4A1 | 44 | 0.13; 0.424 | 0.16; 0.303 | −0.2; 0.208 | 0.13; 0.407 | 0.17; 0.266 | −0.01; 0.935 | 0.04; 0.809 | 0.17; 0.28 |
| NREM alpha C3A2 | 51 | 0; 0.976 | 0.13; 0.373 | −0.13; 0.366 | 0.03; 0.831 | 0.08; 0.574 | 0.09; 0.564 | 0.1; 0.474 | 0.02; 0.891 |
| NREM alpha O2A1 | 40 | 0.18; 0.277 | −0.16; 0.338 | 0.01; 0.942 | −0.01; 0.949 | −0.02; 0.882 | −0.2; 0.238 | 0.16; 0.325 | 0.04; 0.794 |
| NREM alpha O1A2 | 45 | −0.01; 0.96 | −0.01; 0.974 | −0.02; 0.912 | −0.01; 0.97 | −0.06; 0.682 | −0.07; 0.642 | −0.04; 0.806 | 0.01; 0.969 |
| NREM beta F4A1 | 43 | 0.07; 0.649 | 0.10; 0.516 | 0.03; 0.85 | 0.16; 0.323 | −0.12; 0.46 | −0.05; 0.779 | 0.13; 0.428 | 0.25; 0.11 |
| NREM beta F3A2 | 51 | 0.26; 0.068 | −0.12; 0.438 | 0.1; 0.495 | 0.02; 0.881 | 0.11; 0.459 | −0.14; 0.345 | 0; 0.999 | 0.05; 0.748 |
| NREM beta C4A1 | 44 | 0.04; 0.794 | 0.12; 0.447 | −0.13; 0.404 | 0.15; 0.346 | −0.04; 0.815 | 0.01; 0.929 | 0.28; 0.068 | 0.29; 0.068 |
| NREM beta C3A2 | 51 | 0.12; 0.407 | −0.09; 0.554 | −0.02; 0.911 | 0.08; 0.61 | 0.13; 0.366 | −0.01; 0.944 | 0.08; 0.599 | 0.06; 0.667 |
| NREM beta O2A1 | 40 | 0; 0.999 | −0.15; 0.372 | −0.03; 0.849 | 0.08; 0.64 | −0.21; 0.207 | −0.13; 0.453 | 0.31; 0.055 | 0.17; 0.321 |
| NREM beta O1A2 | 45 | −0.09; 0.574 | 0.04; 0.824 | −0.04; 0.8 | 0.05; 0.762 | −0.16; 0.311 | −0.03; 0.873 | 0.06; 0.688 | 0.06; 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sochal, M.; Binienda, A.; Tarasiuk, A.; Gabryelska, A.; Białasiewicz, P.; Ditmer, M.; Turkiewicz, S.; Karuga, F.F.; Fichna, J.; Wysokiński, A. The Relationship between Sleep Parameters Measured by Polysomnography and Selected Neurotrophic Factors. J. Clin. Med. 2024, 13, 893. https://doi.org/10.3390/jcm13030893
Sochal M, Binienda A, Tarasiuk A, Gabryelska A, Białasiewicz P, Ditmer M, Turkiewicz S, Karuga FF, Fichna J, Wysokiński A. The Relationship between Sleep Parameters Measured by Polysomnography and Selected Neurotrophic Factors. Journal of Clinical Medicine. 2024; 13(3):893. https://doi.org/10.3390/jcm13030893
Chicago/Turabian StyleSochal, Marcin, Agata Binienda, Aleksandra Tarasiuk, Agata Gabryelska, Piotr Białasiewicz, Marta Ditmer, Szymon Turkiewicz, Filip Franciszek Karuga, Jakub Fichna, and Adam Wysokiński. 2024. "The Relationship between Sleep Parameters Measured by Polysomnography and Selected Neurotrophic Factors" Journal of Clinical Medicine 13, no. 3: 893. https://doi.org/10.3390/jcm13030893