1. Introduction
Catheter ablation (CA) for atrial fibrillation (AF) has been proven to have the highest efficacy in maintaining sinus rhythm [
1,
2]; however, success rates vary widely, ranging between 29 and 90% [
2,
3,
4]. Not infrequently, cases of very early or late recurrences still occur. Retrospective analyses identified several independent predictors, such as AF type and duration [
5], size of left atrium [
5], older age [
6], female sex [
7], number of antiarrhythmics administered before ablation [
8], higher values of CHADS
2 and CHA
2DS
2-VASc scores [
9], hypertension [
2], obstructive sleep apnea syndrome [
10], coronary atherosclerosis [
4], or metabolic syndrome [
11] to be associated with intervention success. Procedural costs are not negligible, and a low but present rate of severe complications exists. Therefore, an accurate estimation of the results is necessary to assess the risk–benefit ratio. The practice of using prediction scores for management of atrial fibrillation is well known since the thromboembolic risk scores and it is still a relevant tool nowadays, with a trend of developing more specific scores, either for subtypes of AF (as with the FLAME [
12] score) or for specific therapeutic targets (as when predicting LVEF improvement after ablation [
13]). In one of the most recent debates in the field, during the EHRA Congress 2023, Dr. Marco Bergonti of the Cardiocentro Ticino Institute in Lugano, Switzerland, said “further evidence is needed to help stratify and identify those patients who will most likely benefit from atrial fibrillation ablation” as they presented a new score for predicting AF ablation outcomes in a subgroup of patients with heart failure, the ANTWOORD Study [
13]. Utilization of this type of scoring system is helpful in taking a more personalized approach in the decision-making process.
Several other studies have proposed different composite scores for predicting post-procedural success, such as APPLE [
14], CAAP-AF [
15], MB-LATER [
16], and SUCCESS [
17], but most have not been validated in prospective cohorts [
18]. Moreover, some proposed scores included only intra-procedural or post-ablation variables, with poor predictive power and/or low sensitivity/specificity ratios. Therefore, further research is warranted to determine the optimal formula [
18].
The primary objective of our study was to identify independent predictors of AF recurrence after CA, to elaborate an easily calculable score, and to compare it with previously described scores.
2. Materials and Methods
We retrospectively analyzed 263 consecutive patients with paroxysmal or persistent AF who underwent catheter ablation at our center. Preprocedural characteristics (significant comorbidities, cardiovascular risk factors, AF history, and prior medications), ECGs, routine laboratory tests, and echocardiographic data were collected. ECG measurements included the duration and amplitude of the p-wave, suggestive elements of left atrium overload, a notch in DII, or a negative component >40 ms in V1. The “f” fibrillatory waves were divided into 2 categories, lesser and greater than 0.1 mV.
Patients with paroxysmal AF underwent radiofrequency (RF) pulmonary antral vein isolation (PVAI). Entrance and exit pulmonary vein blocks were demonstrated by pacing maneuvers in sinus rhythm (SR). In patients with persistent AF, a stepwise strategy was used. After the initial PVAI, if SR was not restored, additional lines, or activation-guided ablation of the residual atrial tachycardia/flutter was performed until SR was restored. If this was not possible, a chemical or electrical conversion to SR was performed. A cavotricuspid isthmus (CTI) line was created in all patients at the endpoint of a bidirectional conduction block. During the repeat procedure, the persistence of pulmonary vein isolation was evaluated. In the presence of conduction recovery, re-isolation of the PVs was performed using a strategy similar to that of the initial procedure.
During follow-up, screening for arrhythmia recurrence was performed via clinical interview and 48 h Holter monitoring at 1, 3, and 6 months and every 6 months thereafter. A 3-month blanking period was used to define recurrence status. Recurrence was defined as documented AF/atrial tachycardia/atrial flutter on ECG or 48 h Holter monitoring lasting >30 s.
SPSS Statistics version 22 (IBM) and Analyze-It software for Microsoft Excel were used to analyze the data. Continuous variables were presented as mean ± standard deviation or median (IQR), and categorical variables were presented as frequencies. For comparison of subgroups with and without arrhythmia relapse, the Wilcoxon–Mann–Whitney U test, t-test, chi-squared, and Fisher’s exact tests were employed as necessary. Variables that were significantly associated with recurrence risk according to the univariate analysis were introduced into a proportional hazard risk ratio Cox analysis. The cut-off of the p value used for inclusion in the multivariable regression model was p ≤ 0.05. Cox proportional regression using the Enter Forced Method of the tested variables (including multicollinearity testing (tolerance less than 0.1 and VIF value greater than 10)) was used to validate predictors for time to atrial fibrillation recurrence. Performance of the predicted model was assessed by ROC curve and the ROC curve of the model had superior prediction power compared to the ones for each predictor separately. Variables that were statistically significant according to multivariable Cox analysis were used to create a new prediction score after empirically assigning different weights based on the coefficients obtained in the regression and the degree of separation between the Kaplan–Meier curves. Receiver operating characteristic (ROC) curves were generated for a graphical illustration of the performance of the VAT-DHF, CHA2DS2-VASc, and APPLE scores in predicting rhythm outcome, with the area under the curve (AUC) equivalent to the c index for determining the predictive value for a score. The c indices (i.e., areas under the ROC curves) were used to test the new scoring model for predicting post-procedural outcomes.
3. Results
The cohort included 263 patients: 65% male, with a mean age of 55.5 ± 11.7 years, and 61% with paroxysmal AF (161 patients).
The mean follow-up period was 37.6 ± 23.4 months, and the mean procedure number was 1.5 ± 0.8. The mean time from the first AF diagnosis to the first procedure was 4.56 ± 3.79 years, with a minimum of 4 months and a maximum of 22 years. Echocardiographic findings showed that most patients had enlarged atria (left rather than right), with a mean indexed left atrium volume of 37.9 mL (moderate dilation category). The ejection fraction of the left ventricle was most often preserved, with a minimum value of 15%, a maximum of 74%, and a mean of 55%.
The overall success rate at the end of the follow-up period (approximately 3 years) after all procedures was 67%. The univariate analysis of the predictors of recurrence is presented in
Table 1.
Subsequently, the variables that reached statistical significance in the univariate analysis were introduced into the Cox multivariable regression model. The cut-off of the
p value used for inclusion in the multivariable regression model was
p ≤ 0.05. Cox proportional regression using the Enter Forced Method of the tested variables indicated that five of them were independent predictors of recurrence, as shown in
Table 2: persistent subtype of AF, fibrillatory f-waves < 0.1 mV, indexed left atrial volume, presence of type 2 diabetes, and smaller height. Diagnosis of the model: CI for exp (B) 95%, probability for variable entry in the model:
p value less than 0.05, model chi square 52,783, model −2 Log Likelihood 362,369.
ROC analysis showed that patients with a height <170 cm were more likely to experience arrhythmia recurrence (sensitivity of 75% and specificity of 68.2%, AUC = 0.62; 95% CI: 0.51–0.72,
p = 0.026). This was also confirmed by Fisher’s exact test that showed that height <170 cm is associated with an increased risk of recurrence (
p = 0.04). Pearson’s chi-squared test for different left atrium volume categories (
Table 3) was also statistically significant (
p < 0.000). Based on the coefficients obtained in the Cox regression (
Table 2) and the degree of separation of the Kaplan–Meier curves for each predictor over time (graphic representations are available in the
Supplementary Materials), an empirically derived point-based weighting scheme was developed. The variables with the largest separation on the Kaplan–Meier curves over time were assigned the highest number of points in the scoring system. For LAVI, we considered a gradually increasing scheme with one point for each severity class, according to the guideline’s classification of mild/moderate and severe dilatation. The process was facilitated by generating the ROC curves for each of the predictors separately, thus illustrating the increase in prediction power brought by the model score (compared with each predictor individually) as seen in
Figure 1 and
Table 3.
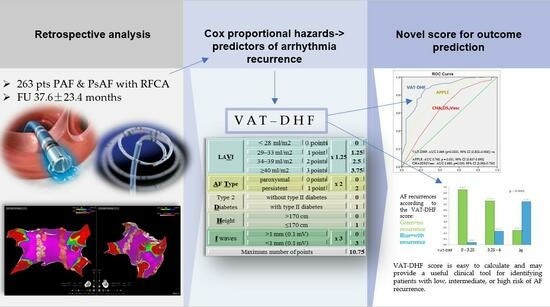
The VAT-DHF scores were calculated as shown in
Table 4. The indexed atrial volume was divided into four categories (according to the echocardiographic classifications of normal, mild, moderate, and severe dilatations).
A cutoff value of 5875 gives the score a sensitivity of 87% and a specificity of 75%. These values, and the AUC of 0.869 (
p < 0.0001, 95% CI [0.802–0.936]), were better than those reported in the literature for similar scores as seen in
Figure 2, for the APPLE score AUC 0.765,
p = 0.001, 95% CI [0.637–0.893] and for the CHA2DS2Vasc AUC 0.655,
p = 0.000, 95% CI [0.580–0.730]. Kaplan–Meier analysis showing arrhythmia-free survival for the two groups of patients with a score above and under the cut-off value is shown in
Figure 3.
Figure 4 shows that the patients who registered a VAT-DHF score between 0 and 3.25, 3.25 and 6, and ≥6 had a 95.7, 76.3, and 25% success rate, respectively, after a mean follow-up period of 37 months.
Testing the new score on a randomly selected sample (50% of the cohort) showed that the predictive value was maintained at an AUC of 0.872 (p < 0.001, 95% CI 0.766–0.942).
4. Discussion
The univariate analysis in our study confirms most of the factors associated with AF recurrence postulated in previous studies, such as the type of persistent fibrillation, hypertension, size of the left atrium, and ischemic heart disease (more precisely, those who underwent a myocardial revascularization procedure). It also showed that some established predictors are not confirmed, such as age and female gender (possibly because the group included a smaller number of women) or are at the limit of significance, for example, obstructive sleep apnea. The presence of valvular heart disease (moderate or severe mitral and tricuspid regurgitation, and moderate or severe aortic stenosis) was strongly associated with the risk of recurrence, as well as other structural heart diseases. Among the electrocardiographic variables, the duration and amplitude of the p-wave were not significantly different between the groups with and without recurrence, but the existence of elements suggestive of left atrial overload (a notch in DII or a negative component >40 ms in V1) exceeded the threshold of statistical significance, and the duration of the QRS complex. The mean QRS duration in the relapsed group was 102.06 ms, while in the non-relapsed group, it was 93.04 ms (p = 0.006), which also correlated with the presence of LBBB, which was higher among those who relapsed (16 vs. 1.4%, p = 0.002).
The main contribution of this study is that it is based on a few clinical and paraclinical variables that are easily obtained and quantified in daily practice, such as AF type, left atrium volume, size of fibrillation f-waves on the EKG, presence of diabetes, and patient height; it is possible to calculate a score for predicting long-term success after AF CA. All individual clinical variables that were identified as independent predictors of relapse in the present study were also found to be associated with post-ablation outcomes in multiple previous studies, but this particular model of combining them offers a very good predictive power, as shown by the ROC analysis. The comparison of predictive power for each of the variables separately and against the new scoring system we developed shows the clear superiority of the latter (
Figure 2). Perhaps one of the less encountered variables associated with recurrence is patient height, for which we found only one previous report that concluded that lower height predisposes to lower success rates [
19]. Although there are few previous reports associating patient height with AF CA outcomes, it is plausible that it plays a significant role given the known association between greater height and a higher incidence of AF [
20]. Therefore, it can be hypothesized that this clinical entity (AF in taller patients) represents a distinct phenotype of AF less connected to risk factors/comorbidities [
21], with a potentially better response to ablative therapy.
Fibrillatory “f” wave size is an easy-to-measure parameter with a sensitivity and specificity of 75% and 73%, respectively, in predicting AF recurrence after ablation [
22]. Some authors [
23] demonstrate that only 12% of those with f > 0.5 mV in V1 and aVF have post-ablation arrhythmic relapses compared to the opposite group.
Elevated LA dimensions are the most commonly reported negative prognostic parameter [
5]. However, a cutoff value that tilts the risk–benefit balance against ablation has not been clearly established. Some authors have proposed an LA diameter >43 mm for inclusion in the APPLE prediction score, a score that was also tested in our study population, but the predictive power was lower than that of the newly formed score, VAT-DHF. A recent meta-analysis [
24] of 33 studies proposing 13 prediction models showed that all evaluated scores had a degree of bias, and the favorable results obtained initially, although very promising, were not reproducible later; therefore, the present results also require confirmation in a larger external cohort.
Other studies have shown that obstructive sleep apnea syndrome is associated with worse outcomes after ablation [
10] and is an independent predictor of recurrence, a fact that was not confirmed by our group, possibly because the general rule in our preablation protocol was that all patients should be screened for sleep apnea syndrome and treated effectively before the procedure.
Several studies [
9,
25] have shown that CHADS
2 and CHA
2DS
2-VASc scores correlate with post-ablation results. However, we did not consider it appropriate to include them in the prediction model because they did not provide independent information. These scores were derived from other parameters, such as age, sex, LV dysfunction, hypertension, and factors that were included individually in the multivariate analysis. Although it was designed to predict thromboembolic risk, given that many of its components have been shown to predict post-ablation AF recurrence, the CHA
2DS
2-VASc score has been used in several studies with modest predictive value. It was also tested in our study population, and similar results were obtained (AUC of 0.65) compared to the AUC of 0.627 described in the literature, which suggests that our population, although not very large, is representative; thereby encouraging testing of the VAT-DHF score on a larger scale.
In this context, our opinion is that this score could help with the appropriate selection of the patients who will benefit the most and be a good tool for prioritization of a limited resource. Also, our experience is that the more information about possible outcomes patients have (especially for non-urgent procedures), the more content they are after the procedure and the more compliant they are to further evaluation and treatment. For example, if a patient with a score higher than six points is informed he has a 75% chance of arrhythmia recurrence but that the arrhythmic burden could still be decreased or even the mortality reduced in some cases, he will perhaps still choose the procedure and not be very disappointed if arrhythmic recurrence occurs.
5. Limitations
Our study had some limitations. The number of patients was limited, and the analysis was conducted retrospectively. Intermittent rhythm monitoring after ablation using only 48 h Holter recording may have underestimated the AF recurrence rate, especially in asymptomatic and undetected AF recurrence. As a future direction, we are planning to verify the new score in a prospective analysis with more cases and external validation. In order to do that, we are now including patients both from our laboratory and from another center in Germany, where one of our fellows is currently in training.
6. Conclusions
In conclusion, the novel VAT-DHF score is easy to calculate and may provide a useful clinical tool for identifying patients with low, intermediate, or high risk of AF recurrence. However, further studies are needed to validate the results.
Author Contributions
A.N.: writing—original draft preparation, writing—review and editing, methodology, validation, formal analysis. Ș.B., C.I., A.D.R. and L.C.-N.: methodology, investigation, resources, data curation; R.G.V.: conceptualization, writing—review and editing, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Clinical Emergency Hospital Bucharest, approval code: 57/2015, on 1 December 2015.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cappato, R.; Calkins, H.; Chen, S.A.; Davies, W.; Iesaka, Y.; Kalman, J.; Kim, Y.-H.; Klein, G.; Natale, A.; Packer, D.; et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, M.; Di Biase, L.; Mohanty, P.; Prasad, S.; Martin, D.; Williams-Andrews, M.; Wazni, O.M.; Burkhardt, J.D.; Cummings, J.E.; Khaykin, Y.; et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: Results from a multicenter study. Heart Rhythm 2009, 6, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Scherr, D.; Khairy, P.; Miyazaki, S.; Aurillac-Lavignolle, V.; Pascale, P.; Wilton, S.B.; Ramoul, K.; Komatsu, Y.; Roten, L.; Jadidi, A.; et al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ. Arrhythm. Electrophysiol. 2015, 8, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Winkle, R.A.; Mead, R.H.; Engel, G.; Patrawala, R.A. Long-term results of atrial fibrillation ablation: The importance of all initial ablation failures undergoing a repeat ablation. Am. Heart J. 2011, 162, 193–200. [Google Scholar] [CrossRef] [PubMed]
- McCready, J.W.; Smedley, T.; Lambiase, P.; Ahsan, S.; Segal, O.; Rowland, E.; Lowe, M.D.; Chow, A.W. Predictors of recurrence following radiofrequency ablation for persistent atrial fibrillation. Europace 2011, 13, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Leong-Sit, P.; Zado, E.; Callans, D.J.; Garcia, F.; Lin, D.; Dixit, S.; Bala, R.; Riley, M.P.; Hutchinson, M.D.; Cooper, J.; et al. Efficacy and risk of atrial fibrillation ablation before 45 years of age. Circ. Arrhythm. Electrophysiol. 2010, 3, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Mohanty, P.; Di Biase, L.; Sanchez, J.; Shaheen, M.; Burkhardt, D.; Bassouni, M.; Cummings, J.; Wang, Y.; Lewis, W.R.; et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm 2010, 7, 167–172. [Google Scholar] [CrossRef]
- Winkle, R.A.; Mead, R.H.; Engel, G.; Kong, M.H.; Patrawala, R.A. Prior antiarrhythmic drug use and the outcome of atrial fibrillation ablation. Europace 2012, 14, 646–652. [Google Scholar] [CrossRef]
- Jacobs, V.; May, H.; Bair, T.; Crandall, B.; Cutler, M.; Day, J.; Weiss, J.P.; Osborn, J.S.; Muhlestein, J.B.; Anderson, J.L.; et al. The impact of risk score (CHADS2 versus CHA2DS2-VASc) on long-term outcomes after atrial fibrillation ablation. Heart Rhythm 2015, 12, 681–686. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Li, J.; Ge, X.; Guo, L.; Wang, Y.; Guo, W.-H.; Jiang, C.-X.; Ma, C.-S. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: A meta-analysis of observational studies. Europace 2014, 16, 1309–1314. [Google Scholar] [CrossRef]
- Mohanty, S.; Mohanty, P.; Di Biase, L.; Bai, R.; Pump, A.; Santangeli, P.; Burkhardt, D.; Gallinghouse, J.G.; Horton, R.; Sanchez, J.E.; et al. Impact of metabolic syndrome on procedural outcomes in patients with atrial fibrillation undergoing catheter ablation. J. Am. Coll. Cardiol. 2012, 59, 1295–12301. [Google Scholar] [CrossRef] [PubMed]
- Boyalla, V.; Jarman, J.W.E.; Markides, V.; Hussain, W.; Wong, T.; Mead, R.H.; Engel, G.; Kong, M.H.; Patrawala, R.A.; Winkle, R.A. Internationally validated score to predict the outcome of non-paroxysmal atrial fibrillation ablation: The ‘FLAME score’. Open Heart 2021, 8, e001653. [Google Scholar] [CrossRef] [PubMed]
- Bergonti, M.; Spera, F.; Tijskens, M.; Bonomi, A.; Saenen, J.; Huybrechts, W.; Miljoen, H.; Wittock, A.; Casella, M.; Tondo, C.; et al. A new prediction model for left ventricular systolic function recovery after catheter ablation of atrial fibrillation in patients with heart failure: The ANTWOORD Study. Int. J. Cardiol. 2022, 358, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Hindricks, G.; Shoemaker, B.; Husser, D.; Arya, A.; Sommer, P.; Rolf, S.; Saavedra, P.; Kanagasundram, A.; Whalen, S.P.; et al. The APPLE score: A novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin. Res. Cardiol. 2015, 104, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Winkle, R.A.; Jarman, J.W.E.; Mead, R.H.; Engel, G.; Kong, M.H.; Fleming, W.; Patrawala, R.A. Predicting atrial fibrillation ablation outcome: The CAAP-AF score. Heart Rhythm 2016, 13, 2119–2125. [Google Scholar] [CrossRef]
- Mujović, N.; Marinković, M.; Marković, N.; Shantsila, A.; Lip, G.Y.H.; Potpara, T.S. Prediction of very late arrhythmia recurrence after radiofrequency catheter ablation of atrial fibrillation: The MB-LATER clinical score. Sci. Rep. 2017, 7, 40828. [Google Scholar] [CrossRef]
- Haegeli, L.M.; Jud, F.N.; Obeid, S.; Duru, F.; Haegeli, L.M. Original Investigation 142 A novel score in the prediction of rhythm outcome after ablation of atrial fibrillation: The SUCCESS score. Anatol. J. Cardiol. Anadolu Kardiyol. Derg. 2019, 21, 142–149. [Google Scholar] [CrossRef]
- Black-Maier, E.; Parish, A.; Steinberg, B.A.; Green, C.L.; Loring, Z.; Barnett, A.S.; Al-Khatib, S.M.; Atwater, B.D.; Daubert, J.P.; Frazier-Mills, C.; et al. Predicting atrial fibrillation recurrence after ablation in patients with heart failure: Validity of the APPLE and CAAP-AF risk scoring systems. Pacing Clin. Electrophysiol. 2019, 42, 1440–1447. [Google Scholar] [CrossRef]
- Thomas, K. Catheter Ablation for Atrial Fibrillation: Predicting Recurrence. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2016. Available online: https://ir.lib.uwo.ca/etd/3791 (accessed on 12 March 2021).
- Rosenberg, M.A.; Patton, K.K.; Sotoodehnia, N.; Karas, M.G.; Kizer, J.R.; Zimetbaum, P.J.; Chang, J.D.; Siscovick, D.; Gottdiener, J.S.; Kronmal, R.A.; et al. The impact of height on the risk of atrial fibrillation: The cardiovascular Health Study. Eur. Heart J. 2012, 33, 2709–2717. [Google Scholar] [CrossRef]
- Rosenberg, M.A.; Kaplan, R.C.; Siscovick, D.S.; Psaty, B.M.; Heckbert, S.R.; Newton-Cheh, C.; Mukamal, K.J. Genetic variants related to height and risk of atrial fibrillation: The cardiovascular health study. Am. J. Epidemiol. 2014, 180, 215–222. [Google Scholar] [CrossRef]
- Cheng, Z.; Deng, H.; Cheng, K.; Chen, T.; Gao, P.; Yu, M.; Fang, Q. The amplitude of fibrillatory waves on leads aVF and V1 predicting the recurrence of persistent atrial fibrillation patients who underwent catheter ablation. Ann. Noninvasive Electrocardiol. 2013, 18, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Nault, I.; Lellouche, N.; Matsuo, S.; Knecht, S.; Wright, M.; Lim, K.; Sacher, F.; Platonov, P.; Deplagne, A.; Bordachar, P.; et al. Clinical value of fibrillatory wave amplitude on surface ECG in patients with persistent atrial fibrillation. J. Interv. Card. Electrophysiol. 2009, 26, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dretzke, J.; Chuchu, N.; Agarwal, R.; Herd, C.; Chua, W.; Fabritz, L.; Bayliss, S.; Kotecha, D.; Deeks, J.J.; Kirchhof, P.; et al. Predicting recurrent atrial fibrillation after catheter ablation: A systematic review of prognostic models. Europace 2020, 22, 748–760. [Google Scholar] [CrossRef]
- Chao, T.; Ambrose, K.; Tsao, H.; Lin, Y.; Chang, S.; Lo, L.; Hu, Y.-F.; Tuan, T.-C.; Suenari, K.; Li, C.-H.; et al. Relationship between the CHADS(2) score and risk of very late recurrences after catheter ablation of paroxysmal atrial fibrillation. Heart Rhythm 2012, 9, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).