Short-Course High-Intensity Statin Treatment during Admission for Myocardial Infarction and LDL-Cholesterol Reduction—Impact on Tailored Lipid-Lowering Therapy at Discharge
Abstract
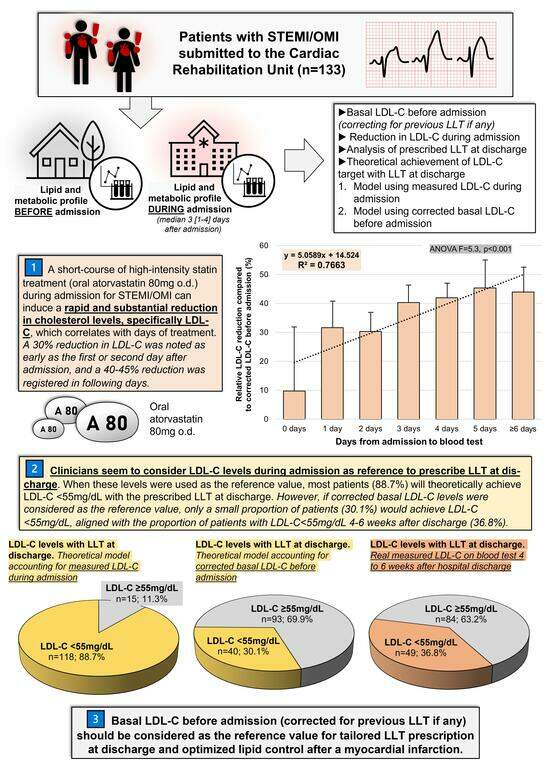
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Cardiac Rehabilitation Program
2.3. Lipid and Metabolic Profile Analysis
2.4. Corrected LDL-C and Potency of LLT
2.5. LLT and LDL-C Reduction during Admission
2.6. LLT at Discharge and LDL-C Levels during Follow-Up
2.7. Objectives of the Study
- To define the efficacy, in a real-world setting, of a short-term course of a high-intensity statin treatment (oral atorvastatin 80 mg o.d.) during admission for MI to reduce LDL-C levels.
- To study the influence of this potential LDL-C reduction compared to basal LDL-C levels in the choice of appropriate LLT at discharge.
2.8. Ethics
2.9. Statistical Analysis
3. Results
3.1. Cohort Description
3.2. Previous LLT and Lipid Profile before Admission
3.3. Lipid Profile before and during Admission
3.4. Days of Admission and LDL-C Reduction
3.5. LLT at Discharge and LDL-C Levels during Follow-Up
4. Discussion
4.1. Lipid Management and Control after MI
4.2. Strategies from Lipidic Control after MI
4.3. Lessons Learned: Optimization of LLT at Discharge
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amini, M.; Zayeri, F.; Salehi, M. Trend Analysis of Cardiovascular Disease Mortality, Incidence, and Mortality-to-Incidence Ratio: Results from Global Burden of Disease Study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Özcan, C.; Deleskog, A.; Schjerning Olsen, A.-M.; Nordahl Christensen, H.; Lock Hansen, M.; Hilmar Gislason, G. Coronary Artery Disease Severity and Long-Term Cardiovascular Risk in Patients with Myocardial Infarction: A Danish Nationwide Register-Based Cohort Study. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, T.; Hasvold, P.; Henriksson, M.; Hjelm, H.; Thuresson, M.; Janzon, M. Cardiovascular Risk in Post-Myocardial Infarction Patients: Nationwide Real World Data Demonstrate the Importance of a Long-Term Perspective. Eur. Heart J. 2015, 36, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment elevationThe Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, J.W.; Weiss, S.R.; Davidson, M.H.; Sprecher, D.L.; Schwartz, S.L.; Lupien, P.-J.; Jones, P.H.; Haber, H.E.; Black, D.M. Reduction of LDL Cholesterol by 25% to 60% in Patients With Primary Hypercholesterolemia by Atorvastatin, a New HMG-CoA Reductase Inhibitor. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 678–682. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health. National Institute for Health. National Institute for Health and Care Excellence Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification. In Evidence Review for Statins: Efficacy and Adverse Effects. NICE Guideline CG181; National Institute for Health and Care Excellence: London, UK, 2023; ISBN 978-1-4731-5205-2. [Google Scholar]
- Fan, A.L.; Fenske, J.N.; Van Harrison, R.; Rubenfire, M.; Marcelino, M.A.; Wells, T.D. UMHS Lipid Therapy Guideline 2020; Quality Department, University of Michigan: Ann Arbor, MI, USA, 2020; Available online: https://michmed-public.policystat.com/policy/8093103/latest/ (accessed on 13 August 2023).
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.P.; Tsang, M.; Wright, J.M. Atorvastatin for Lowering Lipids. Cochrane Database Syst. Rev. 2015, 2017, CD008226. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.P.; Sekhon, S.S.; Wright, J.M. Rosuvastatin for Lowering Lipids. Cochrane Database Syst. Rev. 2014, 2017, CD010254. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.P.; Alaeiilkhchi, N.; Wright, J.M. Pitavastatin for Lowering Lipids. Cochrane Database Syst. Rev. 2020, 2020, CD012735. [Google Scholar] [CrossRef]
- Climent, E.; Bea, A.M.; Benaiges, D.; Brea-Hernando, Á.; Pintó, X.; Suárez-Tembra, M.; Perea, V.; Plana, N.; Blanco-Vaca, F.; Pedro-Botet, J.; et al. LDL Cholesterol Reduction Variability with Different Types and Doses of Statins in Monotherapy or Combined with Ezetimibe. Results from the Spanish Arteriosclerosis Society Dyslipidaemia Registry. Cardiovasc. Drugs Ther. 2022, 36, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Laufs, U.; Ray, K.K.; Leiter, L.A.; Bays, H.E.; Goldberg, A.C.; Stroes, E.S.; MacDougall, D.; Zhao, X.; Catapano, A.L. Bempedoic Acid plus Ezetimibe Fixed-Dose Combination in Patients with Hypercholesterolemia and High CVD Risk Treated with Maximally Tolerated Statin Therapy. Eur. J. Prev. Cardiol. 2020, 27, 593–603. [Google Scholar] [CrossRef]
- Roberts, W.C. The Rule of 5 and the Rule of 7 in Lipid-Lowering by Statin Drugs. Am. J. Cardiol. 1997, 80, 106–107. [Google Scholar] [CrossRef]
- Weng, T.-C.; Yang, Y.-H.K.; Lin, S.-J.; Tai, S.-H. A Systematic Review and Meta-Analysis on the Therapeutic Equivalence of Statins: Therapeutic Equivalence of Statins. J. Clin. Pharm. Ther. 2010, 35, 139–151. [Google Scholar] [CrossRef]
- Burnett, H.; Fahrbach, K.; Cichewicz, A.; Jindal, R.; Tarpey, J.; Durand, A.; Di Domenico, M.; Reichelt, A.; Viljoen, A. Comparative Efficacy of Non-Statin Lipid-Lowering Therapies in Patients with Hypercholesterolemia at Increased Cardiovascular Risk: A Network Meta-Analysis. Curr. Med. Res. Opin. 2022, 38, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Laufs, U.; Ballantyne, C.M.; Banach, M.; Bays, H.; Catapano, A.L.; Duell, P.B.; Goldberg, A.C.; Gotto, A.M.; Leiter, L.A.; Ray, K.K.; et al. Efficacy and Safety of Bempedoic Acid in Patients Not Receiving Statins in Phase 3 Clinical Trials. J. Clin. Lipidol. 2022, 16, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, N.D.; Hanselman, J.C.; MacDougall, D.E.; Sterling, L.R.; Cramer, C.T. Complementary Low-Density Lipoprotein-Cholesterol Lowering and Pharmacokinetics of Adding Bempedoic Acid (ETC-1002) to High-Dose Atorvastatin Background Therapy in Hypercholesterolemic Patients: A Randomized Placebo-Controlled Trial. J. Clin. Lipidol. 2019, 13, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Rubino, J.; MacDougall, D.E.; Sterling, L.R.; Hanselman, J.C.; Nicholls, S.J. Combination of Bempedoic Acid, Ezetimibe, and Atorvastatin in Patients with Hypercholesterolemia: A Randomized Clinical Trial. Atherosclerosis 2021, 320, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Masana, L.; Ibarretxe, D.; Plana, N. Reasons Why Combination Therapy Should Be the New Standard of Care to Achieve the LDL-Cholesterol Targets: Lipid-Lowering Combination Therapy. Curr. Cardiol. Rep. 2020, 22, 66. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-Analysis. JAMA 2016, 316, 1289. [Google Scholar] [CrossRef]
- Trialists, C.T. Cholesterol Treatment Trialists’ (CTT) Collaboration Efficacy and Safety of More Intensive Lowering of LDL Cholesterol: A Meta-Analysis of Data from 170 000 Participants in 26 Randomised Trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Muscoli, S.; Ifrim, M.; Russo, M.; Candido, F.; Sanseviero, A.; Milite, M.; Di Luozzo, M.; Marchei, M.; Sangiorgi, G. Current Options and Future Perspectives in the Treatment of Dyslipidemia. J. Clin. Med. 2022, 11, 4716. [Google Scholar] [CrossRef]
- Elis, A. Current and Future Options in Cholesterol Lowering Treatments. Eur. J. Intern. Med. 2023, 112, 1–5. [Google Scholar] [CrossRef]
- Cannon, C.P.; Steinberg, B.A.; Murphy, S.A.; Mega, J.L.; Braunwald, E. Meta-Analysis of Cardiovascular Outcomes Trials Comparing Intensive Versus Moderate Statin Therapy. J. Am. Coll. Cardiol. 2006, 48, 438–445. [Google Scholar] [CrossRef]
- Kotseva, K.; De Backer, G.; De Bacquer, D.; Rydén, L.; Hoes, A.; Grobbee, D.; Maggioni, A.; Marques-Vidal, P.; Jennings, C.; Abreu, A.; et al. Lifestyle and Impact on Cardiovascular Risk Factor Control in Coronary Patients across 27 Countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V Registry. Eur. J. Prev. Cardiol. 2019, 26, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Pirillo, A.; Farnier, M.; Jukema, J.W.; Laufs, U.; Mach, F.; Masana, L.; Pedersen, T.R.; Schiele, F.; Steg, G.; et al. Lipid-Lowering Therapy and Low-Density Lipoprotein Cholesterol Goal Achievement in Patients with Acute Coronary Syndromes: The ACS Patient Pathway Project. Atheroscler. Suppl. 2020, 42, e49–e58. [Google Scholar] [CrossRef] [PubMed]
- Claessen, B.E.; Guedeney, P.; Gibson, C.M.; Angiolillo, D.J.; Cao, D.; Lepor, N.; Mehran, R. Lipid Management in Patients Presenting With Acute Coronary Syndromes: A Review. J. Am. Heart Assoc. 2020, 9, e018897. [Google Scholar] [CrossRef] [PubMed]
- De Backer, G.; Jankowski, P.; Kotseva, K.; Mirrakhimov, E.; Reiner, Ž.; Rydén, L.; Tokgözoğlu, L.; Wood, D.; De Bacquer, D.; De Backer, G.; et al. Management of Dyslipidaemia in Patients with Coronary Heart Disease: Results from the ESC-EORP EUROASPIRE V Survey in 27 Countries. Atherosclerosis 2019, 285, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.V.; Kosiborod, M.; Tang, F.; Zhao, Z.; Maddox, T.M.; McCollam, P.L.; Birt, J.; Spertus, J.A. Patterns of Statin Initiation, Intensification, and Maximization Among Patients Hospitalized With an Acute Myocardial Infarction. Circulation 2014, 129, 1303–1309. [Google Scholar] [CrossRef]
- Ferrières, J.; Rouyer, M.V.; Lautsch, D.; Ambegaonkar, B.M.; Horack, M.; Brudi, P.; Gitt, A.K. Improvement in Achievement of Lipid Targets in France: Comparison of Data from Coronary Patients in the DYSIS and DYSIS II Studies. Int. J. Cardiol. 2016, 222, 793–794. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Ray, K.K.; Reeskamp, L.F.; Laufs, U.; Banach, M.; Mach, F.; Tokgözoğlu, L.S.; Connolly, D.L.; Gerrits, A.J.; Stroes, E.S.G.; Masana, L.; et al. Combination Lipid-Lowering Therapy as First-Line Strategy in Very High-Risk Patients. Eur. Heart J. 2022, 43, 830–833. [Google Scholar] [CrossRef]
- Makhmudova, U.; Samadifar, B.; Maloku, A.; Haxhikadrija, P.; Geiling, J.-A.; Römer, R.; Lauer, B.; Möbius-Winkler, S.; Otto, S.; Schulze, P.C.; et al. Intensive Lipid-Lowering Therapy for Early Achievement of Guideline-Recommended LDL-Cholesterol Levels in Patients with ST-Elevation Myocardial Infarction (“Jena Auf Ziel”). Clin. Res. Cardiol. 2023, 112, 1212–1219. [Google Scholar] [CrossRef]
- Taylor, R.S.; Dalal, H.M.; McDonagh, S.T.J. The Role of Cardiac Rehabilitation in Improving Cardiovascular Outcomes. Nat. Rev. Cardiol. 2021, 19, 180–194. [Google Scholar] [CrossRef]
- Dibben, G.O.; Faulkner, J.; Oldridge, N.; Rees, K.; Thompson, D.R.; Zwisler, A.-D.; Taylor, R.S. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: A Meta-Analysis. Eur. Heart J. 2023, 44, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Anguita, M.; Arrarte, V.; Barrios, V.; Cequier, Á.; Cosín-Sales, J.; Egocheaga, I.; López De Sa, E.; Masana, L.; Pallarés, V.; et al. Recommendations to Improve Lipid Control. Consensus Document of the Spanish Society of Cardiology. Rev. Esp. Cardiol. 2020, 73, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Antoniou, S.; Cornel, J.H.; Schiele, F.; Perrone-Filardi, P.; Brachmann, J.; Sidelnikov, E.; Villa, G.; Ferguson, S.; Rowlands, C.; et al. European Physician Survey Characterizing the Clinical Pathway and Treatment Patterns of Patients Post-Myocardial Infarction. Adv. Ther. 2023, 40, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Levintow, S.N.; Reading, S.R.; Noshad, S.; Mayer, S.E.; Wiener, C.; Eledath, B.; Exter, J.; Brookhart, M.A. Lipid Testing Trends Before and After Hospitalization for Myocardial Infarction Among Adults in the United States, 2008–2019. Clin. Epidemiol. 2022, 14, 737–748. [Google Scholar] [CrossRef]
- Balci, B. The Modification of Serum Lipids after Acute Coronary Syndrome and Importance in Clinical Practice. Curr. Cardiol. Rev. 2012, 7, 272–276. [Google Scholar] [CrossRef]
- Pitt, B.; Loscalzo, J.; Yčas, J.; Raichlen, J.S. Lipid Levels After Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2008, 51, 1440–1445. [Google Scholar] [CrossRef]
- Barth, J.H.; Jackson, B.M.; Farrin, A.J.; Efthymiou, M.; Worthy, G.; Copeland, J.; Bailey, K.M.; Romaine, S.P.; Balmforth, A.J.; McCormack, T.; et al. Change in Serum Lipids after Acute Coronary Syndromes: Secondary Analysis of SPACE ROCKET Study Data and a Comparative Literature Review. Clin. Chem. 2010, 56, 1592–1598. [Google Scholar] [CrossRef]




| All Patients (n = 133) | No Previous LLT (n = 95) | Previous LLT (n = 38) | p-Value | |
|---|---|---|---|---|
| Clinical variables | ||||
| Age (years) | 62.71 ± 11.3 | 60.82 ± 11 | 67.44 ± 10.7 | 0.002 |
| Male sex (%) | 109 (82) | 83 (87.4) | 26 (68.4) | 0.01 |
| Hypercholesterolemia (%) | 124 (93.2) | 86 (90.5) | 38 (100) | 0.04 |
| Hypertension (%) | 79 (59.4) | 52 (54.7) | 27 (71.1) | 0.08 |
| Diabetes mellitus (%) | 31 (23.3) | 15 (15.8) | 16 (42.1) | 0.001 |
| Smoking habit (%) | 65 (48.9) | 48 (50.5) | 17 (44.7) | 0.55 |
| Killip class ≥ 2 (%) | 39 (29.3) | 25 (26.3) | 14 (36.8) | 0.23 |
| GRACE risk score | 117.66 ± 29.81 | 114.27 ± 29.22 | 126.27 ± 29.93 | 0.04 |
| Anterior infarction (%) | 69 (51.9) | 51 (53.7) | 18 (47.4) | 0.51 |
| LVEF (%) | 52.07 ± 10.6 | 51.41 ± 10.68 | 53.73 ± 10.33 | 0.26 |
| LVEF < 50% (%) | 51 (38.3) | 39 (41.1) | 12 (31.6) | 0.31 |
| Lipid and metabolic profile before admission | ||||
| Fasting blood glucose (mg/dL) | 100.58 ± 22.34 | 98.38 ± 21.34 | 106.03 ± 24.05 | 0.08 |
| Total cholesterol (mg/dL) | 211.32 ± 50.9 | 225.52 ± 45.26 | 175.82 ± 47.2 | <0.001 |
| Triglycerides (mg/dL) | 155.4 ± 91.59 | 162.34 ± 103.11 | 138.18 ± 49.88 | 0.17 |
| HDL-C (mg/dL) | 48.04 ± 10.83 | 48.17 ± 11.06 | 47.71 ± 10.35 | 0.83 |
| Non-HDL-C (mg/dL) | 163.28 ± 48.54 | 177.35 ± 43.19 | 128.11 ± 43.46 | <0.001 |
| LDL-C (mg/dL) | 140.93 ± 41.26 | 153.85 ± 34.8 | 108.63 ± 38.67 | <0.001 |
| Corrected basal LDL-C (mg/dL) # | 161.88 ± 43.43 | 153.85 ± 34.8 | 181.96 ± 55.44 | 0.005 |
| HbA1c (%) * | 6.33 ± 1.26 | 6.09 ± 1.36 | 6.71 ± 1 | 0.11 |
| Lipid and metabolic profile during admission | ||||
| Fasting blood glucose (mg/dL) | 117.85 ± 40.67 | 112.09 ± 32.25 | 132.24 ± 54.43 | 0.04 |
| Total cholesterol (mg/dL) | 162.11 ± 40.66 | 164.97 ± 39.95 | 154.95 ± 42.07 | 0.2 |
| Triglycerides (mg/dL) | 138.02 ± 61.74 | 136.96 ± 66.98 | 140.68 ± 46.81 | 0.75 |
| HDL-C (mg/dL) | 39.52 ± 10.02 | 39.69 ± 10.32 | 39.08 ± 9.37 | 0.75 |
| Non-HDL-C (mg/dL) | 122.59 ± 37.41 | 125.27 ± 36.74 | 115.87 ± 38.73 | 0.19 |
| LDL-C (mg/dL) | 101.71 ± 33.07 | 105.09 ± 31.31 | 93.24 ± 36.16 | 0.06 |
| HbA1c (%) | 6.13 ± 1.11 | 6 ± 1.06 | 6.44 ± 1.19 | 0.06 |
| Lipoprotein (a) (mg/dL) | 52.74 ± 50.07 | 48.45 ± 46.44 | 62.64 ± 57.05 | 0.16 |
| All Patients (n = 133) | No Previous LLT (n = 95) | Previous LLT (n = 38) | Comparison between LLT Groups | ||||
|---|---|---|---|---|---|---|---|
| Lipid and Metabolic Profile Variables | Mean Difference ± SD † | p-Value † | Mean Difference ± SD † | p-Value † | Mean Difference ± SD † | p-Value † | p-Value †† |
| Fasting blood glucose (mg/dL) | 16.96 ± 34.76 | <0.001 | 13.22 ± 28.07 | <0.001 | 26.21 ± 46.63 | 0.001 | 0.12 |
| Total cholesterol (mg/dL) | −49.21 ± 43.01 | <0.001 | −60.55 ± 41 | <0.001 | −20.87 ± 34.27 | 0.001 | <0.001 |
| Triglycerides (mg/dL) | −17.41 ± 79.25 | 0.01 | −25.38 ± 85.26 | 0.005 | 2.5 ± 58.03 | 0.79 | 0.07 |
| HDL-C (mg/dL) | −8.52 ± 8.82 | <0.001 | −8.47 ± 8.95 | <0.001 | −8.63 ± 8.59 | <0.001 | 0.93 |
| Non-HDL-C (mg/dL) | −40.69 ± 41.4 | <0.001 | −52.07 ± 39.03 | <0.001 | −12.24 ± 32.87 | 0.03 | <0.001 |
| LDL-C (mg/dL) | −39.23 ± 34.89 | <0.001 | −48.76 ± 34.24 | <0.001 | −15.4 ± 23.4 | <0.001 | <0.001 |
| LDL-C (mg/dL), compared to corrected basal LDL-C # | −60.18 ± 40.22 | <0.001 | −48.76 ± 34.24 | <0.001 | −88.72 ± 40.28 | <0.001 | <0.001 |
| HbA1c (%) * | 0.22 ± 0.44 | 0.006 | 0.14 ± 0.28 | 0.03 | 0.34 ± 0.61 | 0.06 | 0.28 |
| LLT | Number | Percentage |
|---|---|---|
| Statins | 131 | 98.5% |
| Fluvastatin 80 mg o.d. | 1 | 0.8% |
| Atorvastatin 20 mg o.d. | 1 | 0.8% |
| Atorvastatin 40 mg o.d. | 15 | 11.3% |
| Atorvastatin 60 mg o.d. | 3 | 2.3% |
| Atorvastatin 80 mg o.d. | 49 | 36.8% |
| Rosuvastatin 10 mg o.d. | 4 | 3% |
| Rosuvastatin 15 mg o.d. | 1 | 0.8% |
| Rosuvastatin 20 mg o.d. | 57 | 42.9% |
| Ezetimibe 10 mg o.d. | 70 | 52.6% |
| PCSK9 inhibitors | 1 | 0.8% |
| Fibrates | 3 | 2.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos-Garcés, V.; Merenciano-González, H.; Martínez Mas, M.L.; Palau, P.; Climent Alberola, J.I.; Perez, N.; López-Bueno, L.; Esteban Argente, M.C.; Valls Reig, M.; Muñoz Alcover, R.; et al. Short-Course High-Intensity Statin Treatment during Admission for Myocardial Infarction and LDL-Cholesterol Reduction—Impact on Tailored Lipid-Lowering Therapy at Discharge. J. Clin. Med. 2024, 13, 127. https://doi.org/10.3390/jcm13010127
Marcos-Garcés V, Merenciano-González H, Martínez Mas ML, Palau P, Climent Alberola JI, Perez N, López-Bueno L, Esteban Argente MC, Valls Reig M, Muñoz Alcover R, et al. Short-Course High-Intensity Statin Treatment during Admission for Myocardial Infarction and LDL-Cholesterol Reduction—Impact on Tailored Lipid-Lowering Therapy at Discharge. Journal of Clinical Medicine. 2024; 13(1):127. https://doi.org/10.3390/jcm13010127
Chicago/Turabian StyleMarcos-Garcés, Víctor, Héctor Merenciano-González, María Luz Martínez Mas, Patricia Palau, Josefa Inés Climent Alberola, Nerea Perez, Laura López-Bueno, María Concepción Esteban Argente, María Valls Reig, Raquel Muñoz Alcover, and et al. 2024. "Short-Course High-Intensity Statin Treatment during Admission for Myocardial Infarction and LDL-Cholesterol Reduction—Impact on Tailored Lipid-Lowering Therapy at Discharge" Journal of Clinical Medicine 13, no. 1: 127. https://doi.org/10.3390/jcm13010127