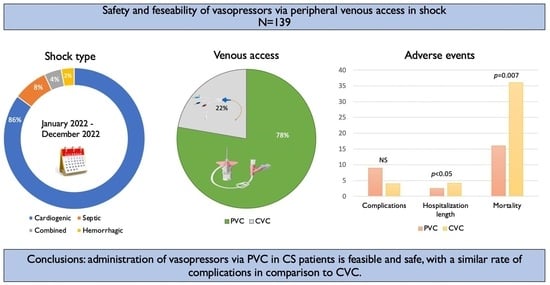
Safety and Outcomes of Peripherally Administered Vasopressor Infusion in Patients Admitted with Shock to an Intensive Cardiac Care Unit—A Single-Center Prospective Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Study Outcomes
2.4. Statistical Methods
3. Results
3.1. Patient Characteristics
3.2. Characteristics of Venous Access and Vasopressor Treatment
3.3. Complications during Admission
3.4. Length of Vasopressor Treatment and Admission
3.5. Mortality Rate
4. Discussion
4.1. Study Limitations
4.2. Summary and Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Müllner, M.; Urbanek, B.; Havel, C.; Losert, H.; Waechter, F.; Gamper, G. Vasopressors for shock. Cochrane Database Syst. Rev. 2004, 3, CD003709. [Google Scholar] [CrossRef]
- Tran, Q.K.; Mester, G.; Bzhilyanskaya, V.; Afridi, L.Z.; Andhavarapu, S.; Alam, Z.; Widjaja, A.; Andersen, B.; Matta, A.; Pourmand, A. Complication of vasopressor infusion through peripheral venous catheter: A systematic review and meta-analysis. Am. J. Emerg. Med. 2020, 38, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Merrer, J.; De Jonghe, B.; Golliot, F.; Lefrant, J.Y.; Raffy, B.; Barre, E.; Rigaud, J.P.; Casciani, D.; Misset, B.; Bosquet, C.; et al. Complications of femoral and subclavian venous catheterization in critically ill patients: A randomized controlled trial. JAMA 2001, 286, 700–707. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.C.; Gould, M.K. Preventing complications of central venous catheterization. N. Engl. J. Med. 2003, 348, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Lennon, M.; Zaw, N.N.; Pöpping, D.M.; Wenk, M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012, 78, 1234–1240. [Google Scholar] [PubMed]
- Kornbau, C.; Lee, K.C.; Hughes, G.D.; Firstenberg, M.S. Central line complications. Int. J. Crit. Illn. Inj. Sci. 2015, 5, 170–178. [Google Scholar] [CrossRef]
- Tian, D.H.; Smyth, C.; Keijzers, G.; Macdonald, S.P.; Peake, S.; Udy, A.; Delaney, A. Safety of peripheral administration of vasopressor medications: A systematic review. Emerg. Med. Australas. 2020, 32, 220–227. [Google Scholar] [CrossRef]
- Bai, X.; Yu, W.; Ji, W.; Lin, Z.; Tan, S.; Duan, K.; Dong, Y.; Xu, L.; Li, N. Early versus delayed administration of norepinephrine in patients with septic shock. Crit. Care 2014, 18, 532. [Google Scholar] [CrossRef]
- Raza, H.A.; Nokes, B.T.; Alvarez, B.; Colquist, J.; Park, J.; Kashyap, R.; Patel, B.; Cartin-Ceba, R. Use of peripherally inserted central catheters with a dedicated vascular access specialists team versus centrally inserted central catheters in the management of septic shock patients in the ICU. J. Vasc. Access. 2022, 11297298221105323. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.L.; et al. Comparison of dopamine and norepinephrine in the treatment of shock. N. Engl. J. Med. 2010, 362, 779–789. [Google Scholar] [CrossRef]
- Bertorello, A.M.; Sznajder, J.I. The dopamine paradox in lung and kidney epithelia: Sharing the same target but operating different signaling networks. Am. J. Respir. Cell Mol. Biol. 2005, 33, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Medlej, K.; Kazzi, A.A.; El Hajj Chehade, A.; Saad Eldine, M.; Chami, A.; Bachir, R.; Zebian, D.; Abou Dagher, G. Complications from Administration of Vasopressors Through Peripheral Venous Catheters: An Observational Study. J. Emerg. Med. 2018, 54, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Loubani, O.M.; Green, R.S. A systematic review of extravasation and local tissue injury from administration of vasopressors through peripheral intravenous catheters and central venous catheters. J. Crit. Care 2015, 30, 653.e9–653.e17. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Surrey, A.; Barmaan, B.; Miller, S.; Oswalt, A.; Evans, D.; Dhindsa, H. Utilization and extravasation of peripheral norepinephrine in the emergency department. Am. J. Emerg. Med. 2021, 39, 55–59. [Google Scholar] [CrossRef]
- Pancaro, C.; Shah, N.; Pasma, W.; Saager, L.; Cassidy, R.; van Klei, W.; Kooij, F.; Vittali, D.; Hollmann, M.W.; Kheterpal, S.; et al. Risk of Major Complications after Perioperative Norepinephrine Infusion through Peripheral Intravenous Lines in a Multicenter Study. Anesth. Analg. 2020, 131, 1060–1065. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Force, S.D.T. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Thiele, H.; Ohman, E.M.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Syed, M.; Patibandla, S.; Sulaiman, S.; Kheiri, B.; Shah, M.K.; Bianco, C.; Balla, S.; Patel, B. Fifteen-Year Trends in Incidence of Cardiogenic Shock Hospitalization and In-Hospital Mortality in the United States. J. Am. Heart Assoc. 2021, 10, e021061. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Khera, S.; Aronow, W.S.; Mujib, M.; Palaniswamy, C.; Sule, S.; Jain, D.; Gotsis, W.; Ahmed, A.; Frishman, W.H.; et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J. Am. Heart Assoc. 2014, 3, e000590. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, N.; Puymirat, E.; Delmas, C.; Ortuno, S.; Durand, E.; Bataille, V.; Drouet, E.; Bonello, L.; Bonnefoy-Cudraz, E.; Lesmeles, G.; et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur. J. Heart Fail. 2020, 22, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Radovanovic, D.; Hunziker, P.R.; Pfisterer, M.E.; Stauffer, J.C.; Erne, P.; Urban, P.; Investigators, A.P.R. Ten-year trends in the incidence and treatment of cardiogenic shock. Ann. Intern. Med. 2008, 149, 618–626. [Google Scholar] [CrossRef]
- Henry, T.D.; Tomey, M.I.; Tamis-Holland, J.E.; Thiele, H.; Rao, S.V.; Menon, V.; Klein, D.G.; Naka, Y.; Piña, I.L.; Kapur, N.K.; et al. Invasive Management of Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e815–e829. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Gore, J.M.; Alpert, J.S.; Osganian, V.; de Groot, J.; Bade, J.; Chen, Z.; Frid, D.; Dalen, J.E. Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. N. Engl. J. Med. 1991, 325, 1117–1122. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Buller, C.E.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef]
- Thiele, H.; de Waha-Thiele, S.; Freund, A.; Zeymer, U.; Desch, S.; Fitzgerald, S. Management of cardiogenic shock. EuroIntervention 2021, 17, 451–465. [Google Scholar] [CrossRef]
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. J. Am. Heart Assoc. 2019, 8, e011991. [Google Scholar] [CrossRef]
- Bruoha, S.; Yosefy, C.; Taha, L.; Dvir, D.; Shuvy, M.; Jubeh, R.; Carasso, S.; Glikson, M.; Asher, E. Mechanical Circulatory Support Devices for the Treatment of Cardiogenic Shock Complicating Acute Myocardial Infarction—A Review. J. Clin. Med. 2022, 11, 5241. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Tascón, G.A.; Hernandez, G.; Alvarez, I.; Calderón-Tapia, L.E.; Manzano-Nunez, R.; Sánchez-Ortiz, A.I.; Quiñones, E.; Ruiz-Yucuma, J.E.; Aldana, J.L.; Teboul, J.L.; et al. Effects of very early start of norepinephrine in patients with septic shock: A propensity score-based analysis. Crit. Care 2020, 24, 52. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, C.B.; Dzavík, V. Inotropes and vasopressors: Review of physiology and clinical use in cardiovascular disease. Circulation 2008, 118, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Avni, T.; Lador, A.; Lev, S.; Leibovici, L.; Paul, M.; Grossman, A. Vasopressors for the Treatment of Septic Shock: Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0129305. [Google Scholar] [CrossRef]
- Lewis, T.; Merchan, C.; Altshuler, D.; Papadopoulos, J. Safety of the Peripheral Administration of Vasopressor Agents. J. Intensive Care Med. 2019, 34, 26–33. [Google Scholar] [CrossRef]
- Levy, B.; Clere-Jehl, R.; Legras, A.; Morichau-Beauchant, T.; Leone, M.; Frederique, G.; Quenot, J.P.; Kimmoun, A.; Cariou, A.; Lassus, J.; et al. Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 173–182. [Google Scholar] [CrossRef]
- Tavazzi, G.; Rossello, X.; Grand, J.; Gierlotka, M.; Sionis, A.; Ahrens, I.; Hassager, C.; Price, S. Epidemiology, monitoring, and treatment strategy in cardiogenic shock. A multinational cross-sectional survey of ESC-acute cardiovascular care association research section. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Gurumurthy, G.; Sridharan, L.; Gupta, D.; Nicholson, W.J.; Jaber, W.A.; Vallabhajosyula, S. A Clinical Update on Vasoactive Medication in the Management of Cardiogenic Shock. Clin. Med. Insights Cardiol. 2022, 16, 11795468221075064. [Google Scholar] [CrossRef] [PubMed]
- Samsky, M.D.; Morrow, D.A.; Proudfoot, A.G.; Hochman, J.S.; Thiele, H.; Rao, S.V. Cardiogenic Shock After Acute Myocardial Infarction: A Review. JAMA 2021, 326, 1840–1850. [Google Scholar] [CrossRef]
| Clinical Variables | All Patients 139 | PVC 108 (78) | CVC 31 (22) | p-Value |
|---|---|---|---|---|
| Age in years (mean ± SD) | 71.6 ± 13.7 | 72 ± 12.3 | 64 ± 19.6 | <0.01 |
| Female sex—No. (%) | 48 (35) | 38 (35) | 10 (32) | NS |
| BMI mean (SD) | 27 | 27 | 27 | NS |
| Hypertension | 90 (65) | 73 (68) | 17 (54) | NS |
| DM | 63 (45) | 46 (43) | 17 (54) | NS |
| Hyperlipidemia | 71 (51) | 55 (51) | 16 (52) | NS |
| Smoking | 23 (17) | 16 (15) | 7 (23) | NS |
| Prior CAD | 58 (42) | 45 (42) | 12 (38) | NS |
| Prior CABG | 13 (9) | 10 (9) | 3 (10) | NS |
| CVA | 15 (11) | 10 (9) | 5 (16) | NS |
| PAD | 9 (6) | 7 (6) | 2 (5) | NS |
| CHF or CMP | 47 (34) | 36 (33) | 11 (35) | NS |
| COPD | 14 (10) | 9 (8) | 5 (16) | NS |
| Atrial fibrillation | 37 (27) | 27 (25) | 10 (32) | NS |
| Anemia | 12 (8.5) | 10 (9) | 2 (5) | NS |
| CKD | 31 (22) | 27 (25) | 4 (13) | NS |
| Shock type | ||||
| Cardiogenic shock | 120 (86) | 91 (84) | 29 (90) | NS |
| Septic shock | 11 (8) | 11 (10) | 0 (0) | |
| Combined | 5 (4) | 3 (3) | 2 (6) | |
| Hemorrhagic | 3 (2) | 3 (3) | 0 (0) |
| Characteristic | Patients (139) |
|---|---|
| Peripheral venous access | 108 (78) |
| PVC location | |
| Above wrist | 108 (100) |
| Gauge | |
| 20 | 108 (100) |
| 18 | 0 (0) |
| Central venous access | 31 (22) |
| CVC location | |
| Jugular | 14 (45) |
| Femoral | 9 (29) |
| Subclavian | 8 (26) |
| All Patients 139 (100) | PVC 108 (78) | CVC 31 (22) | p-Value | |
|---|---|---|---|---|
| Number of vasopressors used | p < 0.01 | |||
| 1 | 110 (79) | 95 (88) | 15 (48) | |
| 2 | 22 (16) | 9 (8) | 13 (42) | |
| >2 | 7 (5) | 4 (4) | 3 (10) | |
| Type of vasopressor | p < 0.01 | |||
| Noradrenaline | 130 (94) | 103 (95) | 27 (87) | |
| Dopamine | 16 (12) | 10 (9) | 6 (19) | |
| Phenylephrine | 13 (9) | 7 (6) | 6 (19) | |
| Vasopressin | 13 (9) | 5 (5) | 8 (26) | |
| Adrenaline | 4 (3) | 2 (2) | 2 (6) | |
| Days of treatment | p < 0.001 | |||
| 1 | 34 (24) | 32 (30) | 2 (6) | |
| 2 | 41 (29) | 32 (30) | 9 (29) | |
| 3 | 26 (19) | 21 (19) | 5 (16) | |
| >3 | 38 (27) | 23 (21) | 15 (48) | |
| Days in ICCU | p < 0.001 | |||
| 1 | 13 (9) | 12 (11) | 1 (3) | |
| 2 | 13 (9) | 8 (7) | 5 (16) | |
| 3 | 12 (9) | 9 (8) | 3 (10) | |
| >3 | 101 (73) | 79 (73) | 22 (71) |
| Patients Treated with Noradrenalin: 130 (94) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Noradrenaline via PVC: 103 (79) | Noradrenaline via CVC: 27 (21) | |||||||||
| Dose (mcg/min) | 1–10 | 11–20 | 21–30 | 31–40 | 41–60 | 1–10 | 11–20 | 21–30 | 31–40 | 41–60 |
| Patients | 48 (47) | 14 (14) | 14 (14) | 5 (5) | 22 (21) | 5 (19) | 2 (7) | 4 (15) | 3 (11) | 13 (48) |
| Duration, mean (days) | 1.83 | 2.7 | 4.5 | 2.8 | 2.9 | 2.2 | 6 | 3.75 | 3.6 | 4.7 |
| Patients | ||||
|---|---|---|---|---|
| Complications | Total (139) | PVC (113) | CVC (31) | p-Value |
| Phlebitis | 7 (5) | 6 (5) | 1 (3) | NS |
| Extravasation | 2 (1) | 1 (1) | 1 (3) | NS |
| Skin necrosis | 0 | 0 | 0 | NS |
| Line sepsis (number/1000 catheter days) | 3 | 2 (2.8) | 1 (3.3) | NS |
| Bleeding | 1 (0.7) | 0 | 1 (3) | NS |
| Pneumothorax | 0 | 0 | 0 | NS |
| Total complications | 13 (9) | 9 (8) | 4 (13) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asher, E.; Karameh, H.; Nassar, H.; Yosefy, C.; Marmor, D.; Perel, N.; Taha, L.; Tabi, M.; Braver, O.; Shuvy, M.; et al. Safety and Outcomes of Peripherally Administered Vasopressor Infusion in Patients Admitted with Shock to an Intensive Cardiac Care Unit—A Single-Center Prospective Study. J. Clin. Med. 2023, 12, 5734. https://doi.org/10.3390/jcm12175734
Asher E, Karameh H, Nassar H, Yosefy C, Marmor D, Perel N, Taha L, Tabi M, Braver O, Shuvy M, et al. Safety and Outcomes of Peripherally Administered Vasopressor Infusion in Patients Admitted with Shock to an Intensive Cardiac Care Unit—A Single-Center Prospective Study. Journal of Clinical Medicine. 2023; 12(17):5734. https://doi.org/10.3390/jcm12175734
Chicago/Turabian StyleAsher, Elad, Hani Karameh, Hamed Nassar, Chaim Yosefy, David Marmor, Nimrod Perel, Louay Taha, Meir Tabi, Omri Braver, Mony Shuvy, and et al. 2023. "Safety and Outcomes of Peripherally Administered Vasopressor Infusion in Patients Admitted with Shock to an Intensive Cardiac Care Unit—A Single-Center Prospective Study" Journal of Clinical Medicine 12, no. 17: 5734. https://doi.org/10.3390/jcm12175734