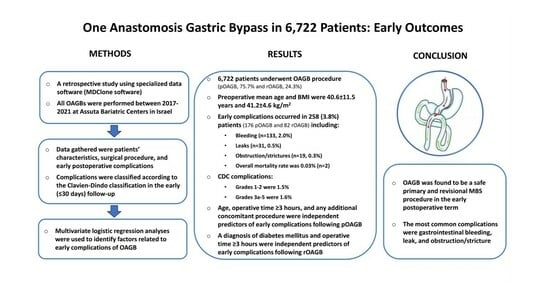
One Anastomosis Gastric Bypass in 6722 Patients: Early Outcomes from a Private Hospital Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Inclusion and Exclusion
2.3. Outcome Measures
2.4. Surgical Technique
2.5. Postoperative Care
2.6. Statistical Analysis
3. Results
3.1. Patient and Surgical Characteristics
3.2. Early Complications
3.3. Early Reoperations and Mortality
4. Discussion
4.1. Operating Time and Length of Stay
4.2. Early Complications
4.3. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrakumar, H.; Khatun, N.; Gupta, T.; Graham-Hill, S.; Zhyvotovska, A.; McFarlane, S.I. The effects of bariatric surgery on cardiovascular outcomes and cardiovascular mortality: A systematic review and meta-analysis. Cureus 2023, 15, e34723. [Google Scholar] [CrossRef]
- Adair, T. Premature cardiovascular disease mortality with overweight and obesity as a risk factor: Estimating excess mortality in the United States during the COVID-19 pandemic. Int. J. Obes. 2023, 47, 273–279. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight Fact Sheet, 9 June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 July 2021).
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Peterli, R.; Wölnerhanssen, B.K.; Peters, T.; Vetter, D.; Kröll, D.; Borbély, Y.; Schultes, B.; Beglinger, C.; Drewe, J.; Schiesser, M.; et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: The SM-BOSS randomized clinical trial. JAMA 2018, 319, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, D.; Wellman, R.; Emiliano, A.; Smith, S.R.; Odegaard, A.O.; Murali, S.; Williams, N.; Coleman, K.J.; Courcoulas, A.; Coley, R.Y.; et al. Comparative effectiveness and safety of bariatric procedures for weight loss: A PCORnet cohort study. Ann. Intern. Med. 2018, 169, 741–750. [Google Scholar] [CrossRef]
- Bhandari, M.; Fobi, M.A.L.; Buchwald, J.N.; Bariatric Metabolic Surgery Standardization (BMSS) Working Group. Standardization of bariatric metabolic procedures: World consensus meeting statement. Obes. Surg. 2019, 29, 309–345. [Google Scholar] [CrossRef]
- García-Caballero, M.; Carbajo, M. One anastomosis gastric bypass: A simple, safe and efficient surgical procedure for treating morbid obesity. Nutr. Hosp. 2004, 19, 372–375. [Google Scholar]
- De Luca, M.; Tie, T.; Ooi, G.; Higa, K.; Himpens, J.; Carbajo, M.A.; Mahawar, K.; Shikora, S.; Brown, W.A. Mini gastric bypass-one anastomosis gastric bypass (MGB-OAGB)-IFSO Position Statement. Obes. Surg. 2018, 28, 1188–1206. [Google Scholar] [CrossRef]
- Landreneau, J.P.; Barajas-Gamboa, J.S.; Strong, A.T.; Corcelles, R.; Kroh, M.D. Conversion of one-anastomosis gastric bypass to Roux-en-Y gastric bypass: Short-term results from a tertiary referral center. Surg. Obes. Relat. Dis. 2019, 15, 1896–1902. [Google Scholar] [CrossRef]
- Brown, W.A.; Kow, L.; Anvari, M.; Ghaferi, A.; Morton, J.; Shikora, S.; Liem, R.; Himpens, J.; Musella, M.; Pattou, F.; et al. Eighth IFSO Global Registry Report; Dendrite Clinical Systems Ltd.: Reading, UK, 2023; Available online: https://www.e-dendrite.com/IFSO8 (accessed on 15 July 2023).
- Kaplan, U.; Romano-Zelekha, O.; Goitein, D.; Keren, D.; Gralnek, I.M.; Boker, L.K.; Sakran, N. Trends in bariatric surgery: A 5-year analysis of the Israel National Bariatric Surgery Registry. Obes. Surg. 2020, 30, 1761–1767. [Google Scholar] [CrossRef]
- Katayama, R.C.; Arasaki, C.H.; Herbella, F.A.M.; Neto, R.A.; Lopes Filho, G.J. One-anastomosis and Roux-en-Y gastric bypass promote similar weight loss, patient satisfaction, quality of life, inflammation grade, and cellular damage in the esophagus and gastric pouch in a short-term follow-up. J. Obes. Metab. Syndr. 2021, 30, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Docimo, S.; Yang, J.; Zhang, X.; Pryor, A.; Spaniolas, K. One anastomosis gastric bypass versus Roux-en-Y gastric bypass: A 30-day follow-up review. Surg. Endosc. 2022, 36, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.G.; Moulla, Y.; Mehdorn, M.; Tuennemann, J.; Zabel-Langhennig, A.; Ouaid, A.; Dietrich, A. Laparoscopic conversion of omega loop gastric bypass to Roux-en-Y gastric bypass for Barrett’s esophagus: Case report. BMC Surg. 2022, 22, 273. [Google Scholar] [CrossRef]
- Kaplan, U.; Aboody-Nevo, H.; Gralnek, I.M.; Sherf-Dagan, S.; Dar, R.; Mokary, S.-E.; Hershko, D.; Kopelman, D.; Sakran, N. Early outcomes and mid-term safety of one anastomosis gastric bypass are comparable with Roux-en-Y gastric bypass: A single center experience. Obes. Surg. 2021, 31, 3786–3792. [Google Scholar] [CrossRef]
- Musella, M.; Susa, A.; Greco, F.; De Luca, M.; Manno, E.; Di Stefano, C.; Milone, M.; Bonfanti, R.; Segato, G.; Antonino, A.; et al. The laparoscopic mini-gastric bypass: The Italian experience: Outcomes from 974 consecutive cases in a multicenter review. Surg. Endosc. 2014, 28, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, J.M.; Arman, G.A.; Guenzi, M.; Rau, C.; Bruzzi, M.; Beaupel, N.; Zinzindohoué, F.; Berger, A. One thousand single anastomosis (omega loop) gastric bypasses to treat morbid obesity in a 7-year period: Outcomes show few complications and good efficacy. Obes. Surg. 2015, 25, 951–958. [Google Scholar] [CrossRef]
- Taha, O.; Abdelaal, M.; Abuzeid, M.; Askalany, A.; All, M. Outcomes of omega loop gastric bypass, 6-years’ experience of 1520 cases. Obes. Surg. 2017, 27, 1952–1960. [Google Scholar] [CrossRef]
- Jung, J.J.; Park, A.K.; Hutter, M.M. The United States Experience with One Anastomosis Gastric Bypass at MBSAQIP-Accredited Centers. Obes. Surg. 2022, 32, 3239–3247. [Google Scholar] [CrossRef]
- Carbajo, M.A.; Luque-de-León, E.; Jiménez, J.M.; Ortiz-de-Solórzano, J.; Pérez-Miranda, M.; Castro-Alija, M.J. Laparoscopic one-anastomosis gastric bypass: Technique, results, and long-term follow-up in 1200 patients. Obes. Surg. 2017, 27, 1153–1167. [Google Scholar] [CrossRef]
- Rheinwalt, K.P.; Plamper, A.; Rückbeil, M.V.; Kroh, A.; Neumann, U.P.; Ulmer, T.F. One Anastomosis Gastric Bypass–Mini-Gastric Bypass (OAGB-MGB) Versus Roux-en-Y Gastric Bypass (RYGB)—A Mid-Term Cohort Study with 612 Patients. Obes. Surg. 2020, 30, 1230–1240. [Google Scholar] [CrossRef]
- Ansar, H.; Zamaninour, N.; Pazouki, A.; Kabir, A. Weight Loss After One Anastomosis Gastric Bypass-Mini Gastric Bypass (OAGB-MGB): Patient-Related Perioperative Predictive Factors. Obes. Surg. 2020, 30, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; EL-Hasani, S. Short- and Mid-term Outcomes of 527 One Anastomosis Gastric Bypass/Mini-Gastric Bypass (OAGB/MGB) Operations: Retrospective Study. Obes. Surg. 2019, 29, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Bariatric Surgery Criteria of the Ministry of Health. The Ministry of Health Website. Available online: https://www.health.gov.il/hozer/mr33_2013.pdf (accessed on 23 June 2023).
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Higa, K.; Himpens, J.; Buchwald, H.; Scopinaro, N. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes. Surg. 2018, 28, 3783–3794. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.D.; Mahawar, K.K. One anastomosis (mini) gastric bypass is now an established bariatric procedure: A systematic review of 12,807 patients. Obes. Surg. 2018, 28, 2956–2967. [Google Scholar] [CrossRef]
- Solouki, A.; Kermansaravi, M.; Jazi, A.H.D.; Kabir, A.; Farsani, T.M.; Pazouki, A. One-anastomosis gastric bypass as an alternative procedure of choice in morbidly obese patients. J. Res. Med. Sci. 2018, 23, 84. [Google Scholar]
- Singhal, R.; Cardoso, V.R.; Wiggins, T.; Super, J.; Ludwig, C.; Gkoutos, G.V.; Mahawar, K.; Pędziwiatr, M.; Major, P.; Zarzycki, P.; et al. 30-day morbidity and mortality of sleeve gastrectomy, Roux-en-Y gastric bypass and one anastomosis gastric bypass: A propensity score-matched analysis of the GENEVA data. Int. J. Obes. 2021, 46, 750–757. [Google Scholar] [CrossRef]
- Parmar, C.D.; Gan, J.; Stier, C.; Dong, Z.; Chiappetta, S.; El-Kadre, L.; Bashah, M.M.; Wang, C.; Sakran, N. One anastomosis/mini gastric bypass (OAGB-MGB) as revisional bariatric surgery after failed primary adjustable gastric band (LAGB) and sleeve gastrectomy (SG): A systematic review of 1075 patients. Int. J. Surg. 2020, 81, 32–38. [Google Scholar] [CrossRef]
- Piazza, L.; Ferrara, F.; Leanza, S.; Coco, D.; Sarvà, S.; Bellia, A.; Di Stefano, C.; Basile, F.; Biondi, A. Laparoscopic mini-gastric bypass: Short-term single-institute experience. Updates Surg. 2011, 63, 239–242. [Google Scholar] [CrossRef]
- Nasta, A.; Goel, R.; Goel, M.; Prasad, A.; Jammu, G.; Fobi, M.; Ismail, M.; Raj, P.; Palaniappan, R.; Aggarwal, S.; et al. Complications after bariatric surgery: A multicentric study of 11,568 patients from Indian bariatric surgery outcomes reporting group. J. Minimal Access Surg. 2021, 17, 213–220. [Google Scholar] [CrossRef]
- Doumouras, A.G.; Saleh, F.; Hong, D. 30-day readmission after bariatric surgery in a publicly funded regionalized center of excellence system. Surg. Endosc. 2016, 30, 2066–2072. [Google Scholar] [CrossRef]
- Bruzzi, M.; Rau, C.; Voron, T.; Guenzi, M.; Berger, A.; Chevallier, J.M. Single anastomosis or mini-gastric bypass: Long-term results and quality of life after a 5-year follow-up. Surg. Obes. Relat. Dis. 2015, 11, 321–326. [Google Scholar] [CrossRef]
- Kermansaravi, M.; Kassir, R.; Valizadeh, R.; Parmar, C.; Jazi, A.H.D.; Shahmiri, S.S.; Benois, M. Management of leaks following one-anastomosis gastric bypass: An updated systematic review and meta-analysis of 44,318 patients. Int. J. Surg. 2023, 109, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, D.; Sergentanis, T.N.; Nixon, A.; Diamantis, T.; Tsigris, C.; Psaltopoulou, T. Efficacy and safety of laparoscopic mini gastric bypass. A systematic review. Surg. Obes. Relat. Dis. 2014, 10, 984–991. [Google Scholar] [CrossRef]
- Smith, M.D.; Adeniji, A.; Wahed, A.S.; Patterson, E.; Chapman, W.; Courcoulas, A.P.; Dakin, G.; Flum, D.; McCloskey, C.; Mitchell, J.E.; et al. Technical factors associated with anastomotic leak after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2015, 11, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Van den Bossche, M.; Kerrigan, D.; Alhamdani, A.; Parmar, C.; Javed, S.; Harper, C.; Darrien, J.; Singhal, R.; Yeluri, S.; et al. Retrospective cohort study of 925 OAGB procedures. The UK MGB/OAGB collaborative group. Int. J. Surg. 2019, 69, 13–18. [Google Scholar] [CrossRef] [PubMed]



| Variable | Total Study Population (n = 6722) | pOAGB Group (n = 5088) | rOAGB Group (n = 1634) | p Value * |
|---|---|---|---|---|
| Age (years), mean ± SD | 40.6 ± 11.5 | 39.6 ± 11.7 | 43.8 ± 10.4 | <0.001 |
| Female, n (%) | 5045 (75.1) | 3814 (75.0) | 1231 (75.3) | 0.768 |
| BMI (kg/m2), mean ± SD | 41.2 ± 4.6 | 41.2 ± 4.5 | 41.2 ± 4.8 | 0.708 |
| Diabetes mellitus, n (%) | 1847 (27.5) | 1401 (27.5) | 446 (27.3) | 0.873 |
| Hypertension, n (%) | 1481 (22.0) | 1071 (21.0) | 410 (25.1) | <0.001 |
| Hyperlipidemia, n (%) | 2501 (37.2) | 1904 (37.4) | 597 (36.5) | 0.537 |
| Obstructive sleep apnea, n (%) | 532 (7.9) | 411 (8.1) | 121 (7.4) | 0.400 |
| Fatty liver disease (NAFLD/NASH), n (%) | 4646 (69.1) | 3606 (70.9) | 1040 (63.6) | <0.001 |
| Gastroesophageal reflux disease, n (%) | 1112 (16.5) | 731 (14.4) | 381 (23.3) | <0.001 |
| Variable | Total Study Population (n = 6722) | pOAGB Group (n = 5088) | rOAGB Group (n = 1634) | p Value * |
|---|---|---|---|---|
| Operative time (minutes), mean ± SD | 67.3 ± 26.6 | 62.7 ± 21.9 | 81.5 ± 33.9 | <0.001 |
| Length of stay (days), mean ± SD (median) | 2.2 ± 1.4 (2.0) | 2.2 ± 0.9 (2.0) | 2.4 ± 2.3 (2.0) | <0.001 |
| Laparoscopic approach, n (%) | 6714 (99.9) | 5086 (99.9) | 1628 (99.6) | >0.999 |
| Patients with previous abdominal surgery, n (%) † | 394 (5.9) | 237 (4.7) | 157 (9.6) | <0.001 |
| Patients with concomitant additional procedures, n (%) | 2122 (31.4) | 898 (17.6) | 1214 (74.3) | <0.001 |
| Total additional procedures, n | 2470 †† | 942 †† | 1528 †† | |
| Gastric band removal, n/N (%) | 945/2470 (38.3) | 0/942 (0.0) | 945/1528 (61.8) | <0.001 |
| Laparoscopic cholecystectomy, n/N (%) | 811/2470 (32.8) | 591/942 (62.7) | 220/1528 (14.4) | 0.029 |
| Hiatal hernia repair, n/N (%) | 534/2470 (21.6) | 300/942 (31.8) | 234/1528 (15.3) | <0.001 |
| Partial gastrectomy, n/N (%) | 99/2470 (4.0) | 0/942 (0.0) | 99/1528 (6.5) | <0.001 |
| Ventral hernia repair, n/N (%) | 81/2470 (3.3) | 51/942 (5.4) | 30/1528 (1.9) | 0.325 |
| Total patients with postoperative complications (some patients had >1 complication) | 258 (3.8) | 176 (3.5) | 82 (5.0) | 0.006 |
| Bleeding, n (%) | 133 (2.0) | 97 (1.9) | 36 (2.2) | 0.475 |
| Leak, n (%) | 31 (0.5) | 19 (0.4) | 12 (0.7) | 0.090 |
| Obstruction/stricture, n (%) | 19 (0.3) | 9 (0.2) | 10 (0.6) | 0.012 |
| Infection, n (%) | 14 (0.2) | 6 (0.1) | 8 (0.5) | 0.009 |
| Respiratory complication, n (%) | 15 (0.2) | 8 (0.2) | 7 (0.4) | 0.064 |
| Small bowel injury, n (%) | 3 (0.04) | 0 (0.0) | 3 (0.2) | 0.014 |
| Acute renal failure, n (%) | 3 (0.04) | 1 (0.02) | 2 (0.1) | 0.148 |
| Readmissions, n (%) | 130 (1.9) | 82 (1.6) | 48 (2.9) | 0.001 |
| Reoperations, n (%) | 61 (0.9) | 39 (0.8) | 22 (1.3) | 0.036 |
| Mortality, n (%) | 2 (0.03) | 1 (0.02) | 1 (0.06) | 0.427 |
| Variable | Previous LAGB rOAGB Patients (n = 1070) | Previous SG rOAGB Patients (n = 420) | Previous SRVG rOAGB Patients (n = 69) | p Value * |
|---|---|---|---|---|
| Operative time (minutes), mean ± SD | 75.3 ± 28.7 | 88.9 ± 34.6 | 121.4 ± 55.3 | <0.001 |
| Length of stay (days), mean ± SD (median) | 2.4 ± 2.1 (2.0) | 2.3 ± 1.1 (2.1) | 2.8 ± 1.4 (2.5) | <0.001 |
| Laparoscopic approach, n (%) | 1067 (99.7) | 419 (99.7) | 68 (98.6) | 0.235 |
| Prior abdominal surgery, n (%) † | 94 (8.8) | 35 (8.3) | 20 (29.0) | <0.001 |
| Concomitant added procedures, n (%) | 939 (87.8) | 165 (39.3) | 54 (78.3) | <0.001 |
| Additional procedures, n | 1155 †† | 211 †† | 54 †† | |
| Gastric band removal, n/N (%) | 889/1155 (76.9) | 0/211 (0.0) | 0/54 (0.0) | <0.001 |
| Laparoscopic cholecystectomy, n/N (%) | 117/1155 (10.1) | 72/211 (34.1) | 12/54 (22.2) | 0.004 |
| Hiatal hernia repair, n/N (%) | 118/1155 (10.2) | 77/211 (36.5) | 19/54 (35.2) | <0.001 |
| Partial gastrectomy, n/N (%) | 14/1155 (1.2) | 54/211 (25.6) | 17/54 (31.5) | <0.001 |
| Ventral hernia repair, n/N (%) | 17/1155 (1.5) | 8/211 (3.8) | 6/54 (11.1) | 0.004 |
| Postoperative complications ** | 53 (5.0) | 17 (4.0) | 7 (10.1) | 0.096 |
| Bleeding, n (%) | 27 (2.5) | 6 (1.4) | 0 (0.0) | 0.191 |
| Leak, n (%) | 7 (0.7) | 1 (0.2) | 2 (2.9) | 0.037 |
| Obstruction/stricture, n (%) | 6 (0.6) | 3 (0.7) | 1 (1.4) | 0.653 |
| Infection, n (%) | 5 (0.5) | 2 (0.5) | 0 (0.0) | 0.850 |
| Respiratory complication, n (%) | 4 (0.4) | 1 (0.2) | 2 (2.9) | 0.007 |
| Small bowel injury, n (%) | 2 (0.2) | 1 (0.2) | 0 (0.0) | 0.914 |
| Acute renal failure, n (%) | 1 (0.1) | 1 (0.2) | 0 (0.0) | 0.747 |
| Readmissions, n (%) | 29 (2.7) | 10 (2.4) | 7 (10.1) | 0.001 |
| Reoperations, n (%) | 15 (1.5) | 4 (1.0) | 1 (1.4) | 0.780 |
| Mortality, n (%) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 0.257 |
| Complications | Total Study Population (n = 6722) | pOAGB Group (n = 5088) | rOAGB Group (n = 1634) | p-Value * |
|---|---|---|---|---|
| Minor | ||||
| CDC grade 1–2, n/N (%) | 104/6722 (1.5) | 77/5088 (1.5) | 27/1634 (1.6) | 0.648 |
| Bleeding, n/N (%) | 84/6722 (1.2) | 62/5088 (1.2) | 22/1634 (1.3) | >0.999 |
| Leaks, n/N (%) | 5/6722 (0.1) | 4/5088 (0.08) | 1/1634 (0.06) | >0.999 |
| Obstruction/stricture, n/N (%) | 0/6722 (0.0) | 0/5088 (0.0) | 0/1634 (0.0) | >0.999 |
| Other †, n/N (%) | 15/6722 (0.2) | 11/5088 (0.2) | 4/1634 (0.2) | >0.999 |
| Major | ||||
| CDC grade 3a-5, n/N (%) | 109/6722 (1.6) | 63/5088 (1.2) | 46/1634 (2.8) | 0.002 |
| Bleeding, n/N (%) | 49/6722 (0.7) | 35/5088 (0.7) | 14/1634 (0.9) | 0.056 |
| Leaks, n/N (%) | 26/6722 (0.4) | 15/5088 (0.3) | 11/1634 (0.6) | 0.842 |
| Obstruction/stricture, n/N (%) | 19/6722 (0.2) | 9/5088 (0.2) | 10/1634 (0.6) | 0.189 |
| Other †, n/N (%) | 13/6722 (0.2) | 3/5088 (0.06) | 10/1634 (0.6) | 0.004 |
| Death, n/N (%) | 2/6722 (0.03) †† | 1/5088 (0.01) | 1/1634 (0.06) | >0.999 |
| Factor | Patients without Complications (n = 4912) | Patients with Complications * (n = 176) | p-Value |
|---|---|---|---|
| Female, n (%) | 3688 (75.1) | 126 (71.2) | 0.289 |
| Age (years), mean ± SD | 39.5 ± 11.6 | 41.7 ± 12.9 | 0.027 |
| Age ≥ 60 years, n (%) | 214 (4.4) | 18 (10.2) | 0.001 |
| BMI (kg/m2), mean ± SD | 41.2 ± 4.5 | 40.8 ± 4.5 | 0.143 |
| Baseline BMI (kg/m2) ≥ 50 kg/m2, n (%) | 220 (4.9) | 8 (5.3) | 0.847 |
| Smoker, n (%) | 284 (5.8) | 10 (5.7) | >0.999 |
| Operative length (minutes), mean ± SD | 62.4 ± 21.4 | 70.9 ± 32.5 | <0.001 |
| Operative length ≥ 3 h, n (%) | 11 (0.2) | 2 (1.2) | 0.073 |
| Additional concomitant procedures (%yes) †, n (%) | 853 (17.4) | 45 (25.6) | 0.009 |
| Lap. cholecystectomy, n (%) | 560 (11.4) | 31 (17.6) | 0.016 |
| Lap. hiatal hernia repair, n (%) | 289 (5.9) | 11 (6.3) | 0.746 |
| Lap. ventral hernia repair, n (%) | 46 (0.9) | 5 (2.8) | 0.030 |
| Associated medical problems | |||
| Hypertension, n (%) | 1031 (21.0) | 40 (22.7) | 0.573 |
| Diabetes mellitus, n (%) | 1351 (27.5) | 50 (28.4) | 0.797 |
| Hyperlipidemia, n (%) | 1833 (37.3) | 71 (40.3) | 0.428 |
| Obstructive Sleep Apnea, n (%) | 398 (8.1) | 13 (7.4) | 0.888 |
| Fatty liver disease, n (%) | 3491 (71.1) | 115 (65.3) | 0.109 |
| Aspirin treatment, n (%) | 199 (4.1) | 12 (6.8) | 0.081 |
| Hypercoagulopathy, n (%) | 51 (1.0) | 1 (0.6) | >0.999 |
| Pulmonary embolism, n (%) | 5 (0.1) | 0 (0.0) | >0.999 |
| Pseudotumor cerebri, n (%) | 20 (0.4) | 1 (0.6) | 0.523 |
| Psychiatric disease, n (%) | 67 (1.4) | 2 (1.1) | >0.999 |
| Depression, n (%) | 490 (10.0) | 19 (10.8) | 0.701 |
| Previous abdominal operation, n (%) | 229 (4.7) | 8 (4.5) | >0.999 |
| Factor | Patients without Complications (n = 1552) | Patients with Complications * (n = 82) | p-Value |
|---|---|---|---|
| Female, n (%) | 1172 (75.5) | 59 (72.0) | 0.511 |
| Age (years), mean ± SD | 43.7 ± 10.3 | 46.7 ± 10.0 | 0.018 |
| Age ≥ 60 years, n (%) | 87 (5.6) | 7 (8.5) | 0.324 |
| BMI (kg/m2), mean ± SD | 41.2 ± 4.8 | 41.4 ± 4.6 | 0.621 |
| Baseline BMI (kg/m2) ≥ 50, n (%) | 63 (4.9) | 2 (3.6) | >0.999 |
| Smoker, n (%) | 126 (8.1) | 9 (11.0) | 0.406 |
| Operative length (minutes), mean ± SD | 81.0 ± 32.9 | 89.9 ± 50.9 | 0.809 |
| Operative length ≥ 3 h, n (%) | 28 (1.9) | 7 (9.9) | <0.001 |
| Additional concomitant procedures (%yes) †, n (%) | 1552 (73.9) | 67 (81.7) | 0.121 |
| Lap. removal of gastric band, n (%) | 894 (57.6) | 51 (62.2) | 0.425 |
| Lap. cholecystectomy, n (%) | 207 (13.3) | 13 (15.9) | 0.507 |
| Lap. hiatal hernia repair, n (%) | 221 (14.2) | 13 (15.9) | 0.630 |
| Lap. partial gastrectomy, n (%) | 88 (5.7) | 11 (13.4) | 0.014 |
| Lap. ventral hernia repair, n (%) | 26 (1.7) | 4 (4.9) | 0.060 |
| Previous bariatric procedure | |||
| Lap. adjustable band, n (%) | 1118 (72.0) | 64 (78.0) | 0.257 |
| Lap. sleeve gastrectomy, n (%) | 473 (30.5) | 22 (26.8) | 0.539 |
| Vertical banded gastroplasty, n (%) | 74 (4.8) | 9 (11.0) | 0.033 |
| Associated medical problems | |||
| Hypertension, n (%) | 390 (25.1) | 20 (24.4) | >0.999 |
| Diabetes mellitus, n (%) | 419 (27.0) | 27 (32.9) | 0.253 |
| Hyperlipidemia, n (%) | 565 (36.4) | 32 (39.0) | 0.639 |
| Obstructive Sleep Apnea, n (%) | 113 (7.3) | 8 (9.8) | 0.385 |
| Fatty liver disease, n (%) | 988 (63.7) | 52 (63.4) | >0.999 |
| Aspirin treatment, n (%) | 69 (4.4) | 3 (3.7) | >0.999 |
| Hypercoagulopathy, n (%) | 13 (0.8) | 1 (1.2) | 0.515 |
| Pulmonary embolism, n (%) | 3 (0.2) | 0 (0.0) | >0.999 |
| Pseudotumor cerebri, n (%) | 6 (0.4) | 0 (0.0) | >0.999 |
| Psychiatric disease, n (%) | 19 (1.2) | 1 (1.2) | >0.999 |
| Depression, n (%) | 196 (12.6) | 9 (11.0) | 0.864 |
| Previous abdominal operation, n (%) | 146 (9.4) | 11 (13.4) | 0.246 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakran, N.; Sherf-Dagan, S.; Hod, K.; Kaplan, U.; Azaria, B.; Raziel, A.; Assuta Bariatric Surgeons Collaborative. One Anastomosis Gastric Bypass in 6722 Patients: Early Outcomes from a Private Hospital Registry. J. Clin. Med. 2023, 12, 6872. https://doi.org/10.3390/jcm12216872
Sakran N, Sherf-Dagan S, Hod K, Kaplan U, Azaria B, Raziel A, Assuta Bariatric Surgeons Collaborative. One Anastomosis Gastric Bypass in 6722 Patients: Early Outcomes from a Private Hospital Registry. Journal of Clinical Medicine. 2023; 12(21):6872. https://doi.org/10.3390/jcm12216872
Chicago/Turabian StyleSakran, Nasser, Shiri Sherf-Dagan, Keren Hod, Uri Kaplan, Bella Azaria, Asnat Raziel, and Assuta Bariatric Surgeons Collaborative. 2023. "One Anastomosis Gastric Bypass in 6722 Patients: Early Outcomes from a Private Hospital Registry" Journal of Clinical Medicine 12, no. 21: 6872. https://doi.org/10.3390/jcm12216872