Epicardial Adipose Tissue and Atrial Fibrillation Recurrence following Catheter Ablation: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias
2.5. Statistical Analysis
3. Results
3.1. Search Results and Studies Characteristics
3.2. Bias Assessment
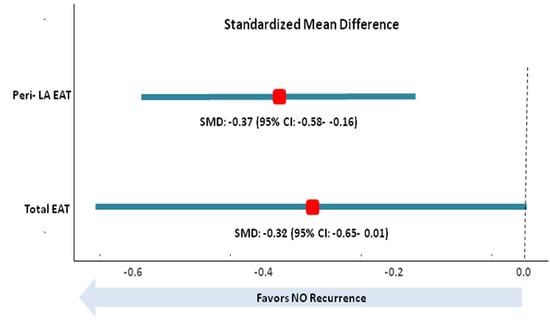
3.3. Total EAT and AF Recurrence
3.4. Peri-LA EAT and AF Recurrence
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kannel, W.B.; Abbott, R.D.; Savage, D.D.; McNamara, P.M. Epidemiologic features of chronic atrial fibrillation: The Framingham study. N. Engl. J. Med. 1982, 306, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.; Franco, O.H.; Hofman, A.; Witteman, J.C.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union. from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef]
- Gómez-Outes, A.; Lagunar-Ruíz, J.; Terleira-Fernández, A.I.; Calvo-Rojas, G.; Suárez-Gea, M.L.; Vargas-Castrillón, E. Causes of Death in Anticoagulated Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2016, 68, 2508–2521. [Google Scholar] [CrossRef] [PubMed]
- Pokorney, S.D.; Piccini, J.P.; Stevens, S.R.; Patel, M.R.; Pieper, K.S.; Halperin, J.L.; Breithardt, G.; Singer, D.E.; Hankey, G.J.; Hacke, W.; et al. ROCKET AF Steering Committee & Investigators; ROCKET AF Steering Committee Investigators. Cause of Death and Predictors of All-Cause Mortality in Anticoagulated Patients WithNonvalvular Atrial Fibrillation: Data from ROCKET AF. J. Am. Heart Assoc. 2016, 5, e002197. [Google Scholar]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Ganesan, A.N.; Shipp, N.J.; Brooks, A.G.; Kuklik, P.; Lau, D.H.; Lim, H.S.; Sullivan, T.; Roberts-Thomson, K.C.; Sanders, P. Long-term outcomes of catheter ablation of atrial fibrillation: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004549. [Google Scholar] [CrossRef] [PubMed]
- Sacks, H.S.; Fain, J.N. Human epicardial adipose tissue: A review. Am. Heart J. 2007, 153, 907–917. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Wang, C.P.; Hsu, H.L.; Hung, W.C.; Yu, T.H.; Chen, Y.H.; Chiu, C.A.; Lu, L.F.; Chung, F.M.; Shin, S.J.; Lee, Y.J. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin. Endocrinol. 2009, 70, 876–882. [Google Scholar] [CrossRef]
- Taguchi, R.; Takasu, J.; Itani, Y.; Yamamoto, R.; Yokoyama, K.; Watanabe, S.; Masuda, Y. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis 2001, 157, 203–209. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Shi, R.; Beck, S.R.; Langefeld, C.D.; Lenchik, L.; Wagenknecht, L.E.; Freedman, B.I.; Rich, S.S.; Bowden, D.W.; Chen, M.Y.; et al. Pericardial and visceral adipose tissues measured volumetrically with computed tomography are highly associated in type 2 diabetic families. Invest. Radiol. 2005, 40, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Thanassoulis, G.; Massaro, J.M.; O’Donnell, C.J.; Hoffmann, U.; Levy, D.; Ellinor, P.T.; Wang, T.J.; Schnabel, R.B.; Vasan, R.S.; Fox, C.S.; et al. Pericardial fat is associated with prevalent atrial fibrillation: The Framingham Heart Study. Circ. Arrhythmia Electrophysiol. 2010, 3, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Al Chekakie, M.O.; Welles, C.C.; Metoyer, R.; Ibrahim, A.; Shapira, A.R.; Cytron, J.; Santucci, P.; Wilber, D.J.; Akar, J.G. Pericardial fat is independently associated with human atrial fibrillation. J. Am. Coll. Cardiol. 2010, 56, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.N.; Redheuil, A.; Gandjbakhch, E. Cardiac adipose tissue and atrial fibrillation: The perils of adiposity. Cardiovasc. Res. 2016, 109, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Fukuda, S.; Tanaka, A.; Otsuka, K.; Sakamoto, M.; Taguchi, H.; Yoshikawa, J.; Shimada, K.; Yoshiyama, M. Peri-atrial epicardial adipose tissue is associated with new-onset nonvalvular atrial fibrillation. Circ. J. 2012, 76, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Yorgun, H.; Canpolat, U.; Aytemir, K.; Hazırolan, T.; Şahiner, L.; Kaya, E.B.; Kabakci, G.; Tokgözoğlu, L.; Özer, N.; Oto, A. Association of epicardial and peri-atrial adiposity with the presence and severity of non-valvular atrial fibrillation. Int. J. Cardiovasc. Imaging 2015, 31, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Markman, T.M.; Khoshknab, M.; Nazarian, S. Catheter ablation of atrial fibrillation: Cardiac imaging guidance as an adjunct to the electrophysiological guided approach. Europace 2021, 23, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Tokarska, L.; Stühlinger, M.; Feuchtner, G.; Hintringer, F.; Honold, S.; Fiedler, L.; Schönbauer, M.S.; Schönbauer, R.; Plank, F. Structural Cardiac Remodeling in Atrial Fibrillation. JACC Cardiovasc. Imaging 2021, 14, 2199–2208. [Google Scholar] [CrossRef]
- Kawasaki, M.; Yamada, T.; Furukawa, Y.; Morita, T.; Tamaki, S.; Kida, H.; Sakata, Y.; Fukunami, M. Are cardiac sympathetic nerve activity and epicardial adipose tissue associated with atrial fibrillation recurrence after catheter ablation in patients without heart failure? Int. J. Cardiol. 2020, 303, 41–48. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- National Institutes of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 26 September 2022).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple. graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.; Cochrane Consumers and Communication Review Group. Heterogeneity and Subgroup Analyses in Cochrane Consumers and Communication Group Reviews: Planning the Analysis at Protocol Stage. Available online: https://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/public/uploads/heterogeneity_subgroup_analyses_revising_december_1st_2016.pdf (accessed on 26 August 2022).
- Nagashima, K.; Okumura, Y.; Watanabe, I.; Nakai, T.; Ohkubo, K.; Kofune, T.; Kofune, M.; Mano, H.; Sonoda, K.; Hirayama, A. Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ. J. 2011, 75, 2559–2565. [Google Scholar] [CrossRef]
- Tsao, H.M.; Hu, W.C.; Wu, M.H.; Tai, C.T.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Wu, T.J.; et al. Quantitative analysis of quantity and distribution of epicardial adipose tissue surrounding the left atrium in patients with atrial fibrillation and effect of recurrence after ablation. Am. J. Cardiol. 2011, 107, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Hori, Y.; Kobayashi, S.; Sakai, Y.; Taguchi, I.; Takayanagi, K.; Nagashima, K.; Sonoda, K.; Kogawa, R.; Sasaki, N.; et al. Epicardial adipose tissue-based defragmentation approach to persistent atrial fibrillation: Its impact on complex fractionated electrograms and ablation outcome. Heart Rhythm 2014, 11, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kumagai, K.; Minami, K.; Nakano, M.; Inoue, H.; Oshima, S. Location of epicardial adipose tissue affects the efficacy of a combined dominant frequency and complex fractionated atrial electrogram ablation of atrial fibrillation. Heart Rhythm 2015, 12, 257–265. [Google Scholar] [CrossRef]
- Masuda, M.; Mizuno, H.; Enchi, Y.; Minamiguchi, H.; Konishi, S.; Ohtani, T.; Yamaguchi, O.; Okuyama, Y.; Nanto, S.; Sakata, Y. Abundant epicardial adipose tissue surrounding the left atrium predicts early rather than late recurrence of atrial fibrillation after catheter ablation. J. Interv. Card. Electrophysiol. 2015, 44, 31–37. [Google Scholar] [CrossRef]
- Singhal, R.; Lo, L.W.; Lin, Y.J.; Chang, S.L.; Hu, Y.F.; Chao, T.F.; Chung, F.P.; Chiou, C.W.; Tsao, H.M.; Chen, S.A. Intrinsic Cardiac Autonomic Ganglionated Plexi within Epicardial Fats Modulate the Atrial Substrate Remodeling: Experiences with Atrial Fibrillation Patients Receiving Catheter Ablation. Acta Cardiol. Sin. 2016, 32, 174–184. [Google Scholar] [PubMed]
- Monno, K.; Okumura, Y.; Saito, Y.; Aizawa, Y.; Nagashima, K.; Arai, M.; Watanabe, R.; Wakamatsu, Y.; Otsuka, N.; Yoda, S.; et al. Effect of epicardial fat and metabolic syndrome on reverse atrial remodeling after ablation for atrial fibrillation. J. Arrhythm. 2018, 34, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Sakamoto, T.; Yamaguchi, Y.; Tsujino, Y.; Kinugawa, K. Epicardial adipose tissue affects the efficacy of left atrial posterior wall isolation for persistent atrial fibrillation. J. Arrhythm. 2020, 36, 652–659. [Google Scholar] [CrossRef]
- Hammache, N.; Pegorer-Sfes, H.; Benali, K.; Magnin Poull, I.; Olivier, A.; Echivard, M.; Pace, N.; Minois, D.; Sadoul, N.; Mandry, D.; et al. Is There an Association between Epicardial Adipose Tissue and Outcomes after Paroxysmal Atrial Fibrillation Catheter Ablation? J. Clin. Med. 2021, 10, 3037. [Google Scholar] [CrossRef]
- Goldenberg, G.R.; Hamdan, A.; Barsheshet, A.; Neeland, I.; Kadmon, E.; Yavin, H.; Omelchenko, A.; Erez, A.; Marcuschamer, I.; Kornowski, R.; et al. Epicardial fat and the risk of atrial tachy-arrhythmia recurrence post pulmonary vein isolation: A computed tomography study. Int. J. Cardiovasc. Imaging 2021, 37, 2785–2790. [Google Scholar] [CrossRef]
- Yang, M.; Cao, Q.; Xu, Z.; Ge, Y.; Li, S.; Yan, F.; Yang, W. Development and Validation of a Machine Learning-Based Radiomics Model on Cardiac Computed Tomography of Epicardial Adipose Tissue in Predicting Characteristics and Recurrence of Atrial Fibrillation. Front. Cardiovasc. Med. 2022, 9, 813085. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.; Li, Z.; Wang, J.; Zhang, C. Correlation analysis between heart rate variability. epicardial fat thickness. visfatin and AF recurrence post radiofrequency ablation. BMC Cardiovasc. Disord. 2022, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Bao, W.; Xu, Z.; Qin, L.; Zhang, N.; Yan, F.; Yang, W. Association between epicardial adipose tissue and recurrence of atrial fibrillation after ablation: A propensity score-matched analysis. Int. J. Cardiovasc. Imaging 2022, 38, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Sepehri Shamloo, A.; Dagres, N.; Dinov, B.; Sommer, P.; Husser-Bollmann, D.; Bollmann, A.; Hindricks, G.; Arya, A. Is epicardial fat tissue associated with atrial fibrillation recurrence after ablation? A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2019, 22, 132–138. [Google Scholar] [CrossRef]
- Chen, J.; Mei, Z.; Yang, Y.; Dai, C.; Wang, Y.; Zeng, R.; Liu, Q. Epicardial adipose tissue is associated with higher recurrence risk after catheter ablation in atrial fibrillation patients: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2022, 22, 264. [Google Scholar] [CrossRef]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2015, 36, 795–805a. [Google Scholar] [CrossRef]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef]
- Parisi, V.; Rengo, G.; Pagano, G.; D’Esposito, V.; Passaretti, F.; Caruso, A.; Grimaldi, M.G.; Lonobile, T.; Baldascino, F.; De Bellis, A.; et al. Epicardial adipose tissue has an increased thickness and is a source of inflammatory mediators in patients with calcific aortic stenosis. Int. J. Cardiol. 2015, 186, 167–169. [Google Scholar] [CrossRef]
- Kremen, J.; Dolinkova, M.; Krajickova, J.; Blaha, J.; Anderlova, K.; Lacinova, Z.; Haluzikova, D.; Bosanska, L.; Vokurka, M.; Svacina, S.; et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: Possible role in postoperative insulin resistance. J. Clin. Endocrinol. Metab. 2006, 91, 4620–4627. [Google Scholar] [CrossRef] [PubMed]
- Suffee, N.; Moore-Morris, T.; Jagla, B.; Mougenot, N.; Dilanian, G.; Berthet, M.; Proukhnitzky, J.; Le Prince, P.; Tregouet, D.A.; Pucéat, M.; et al. Circ. Res. 2020, 126, 1330–1342. [Google Scholar]
- Iacobellis, G.; Leonetti, F.; Singh, N.M.; Sharma, A. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int. J. Cardiol. 2007, 115, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Harrop, D.L.; Ng, A.C.T.; Wang, W.Y.S. Epicardial Adipose Tissue Is Associated with Left Atrial Dysfunction in People Without Obstructive Coronary Artery Disease or Atrial Fibrillation. Can. J. Cardiol. 2018, 34, 1019–1025. [Google Scholar] [CrossRef]
- Wu, N.; Xu, B.; Xiang, Y.; Wu, L.; Zhang, Y.; Ma, X.; Tong, S.; Shu, M.; Song, Z.; Li, Y.; et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: A meta-analysis. Int. J. Cardiol. 2013, 169, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, I.; Kousta, M.; Kossyvakis, C.; Lakka, E.; Paraskevaidis, N.T.; Schizas, N.; Deftereos, S.; Giannopoulos, G. The role of left atrial peak systolic strain in atrial fibrillation recurrence after catheter ablation. A systematic review and meta-analysis. Acta Cardiol. 2021, 20, 1–9. [Google Scholar] [CrossRef]
- Lin, Y.K.; Chen, Y.C.; Chen, J.H.; Chen, S.A.; Chen, Y.J. Adipocytes modulate the electrophysiology of atrial myocytes: Implications in obesity-induced atrial fibrillation. Basic Res. Cardiol. 2012, 107, 293. [Google Scholar] [CrossRef]
- Rook, M.B.; Jongsma, H.J.; de Jonge, B. Single channel currents of homo- and heterologous gap junctions between cardiac fibroblasts and myocytes. Pflug. Arch. 1989, 414, 95–98. [Google Scholar] [CrossRef]
- Miragoli, M.; Gaudesius, G.; Rohr, S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ. Res. 2006, 98, 801–810. [Google Scholar] [CrossRef]
- Friedman, D.J.; Wang, N.; Meigs, J.B.; Hoffmann, U.; Massaro, J.M.; Fox, C.S.; Magnani, J.W. Pericardial fat is associated with atrial conduction: The Framingham Heart Study. J. Am. Heart Assoc. 2014, 3, e000477. [Google Scholar] [CrossRef]
- Jhuo, S.J.; Hsieh, T.J.; Tang, W.H.; Tsai, W.C.; Lee, K.T.; Yen, H.W.; Lai, W.T. The association of the amounts of epicardial fat. P wave duration. and PR interval in electrocardiogram. J. Electrocardiol. 2018, 51, 645–651. [Google Scholar] [PubMed]
- Quisi, A.; Şentürk, S.E.; Harbalıoğlu, H.; Baykan, A.O. The relationship between echocardiographic epicardial adipose tissue. P-wave dispersion. and corrected QT interval. Turk. Kardiyol. Dern. Ars. 2018, 46, 471–478. [Google Scholar] [PubMed]
- Kanazawa, H.; Yamabe, H.; Enomoto, K.; Koyama, J.; Morihisa, K.; Hoshiyama, T.; Matsui, K.; Ogawa, H. Importance of pericardial fat in the formation of complex fractionated atrial electrogram region in atrial fibrillation. Int. J. Cardiol. 2014, 174, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Bandera, F.; Tassinari, F.; Capasso, L.; Cargnelutti, M.; Pelissero, G.; Malavazos, A.E.; Ricci, C. Is epicardial fat depot associated with atrial fibrillation? A systematic review and meta-analysis. Europace 2017, 19, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. EAST-AFNET 4 Trial Investigators. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. New Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality. Stroke. Bleeding. and Cardiac Arrest Among Patients with Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef]
- Pallisgaard, J.L.; Gislason, G.H.; Hansen, J.; Johannessen, A. Torp-Pedersen C. Rasmussen PV. Hansen ML. Temporal trends in atrial fibrillation recurrence rates after ablation between 2005 and 2014: A nationwide Danish cohort study. Eur. Heart J. 2018, 39, 442–449. [Google Scholar] [CrossRef]
- Savelieva, I.; Kakouros, N.; Kourliouros, A.; Camm, A.J. Upstream therapies for management of atrial fibrillation: Review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: Primary prevention. Europace 2011, 13, 308–328. [Google Scholar]
- Pokushalov, E.; Kozlov, B.; Romanov, A.; Strelnikov, A.; Bayramova, S.; Sergeevichev, D.; Bogachev-Prokophiev, A.; Zheleznev, S.; Shipulin, V.; Lomivorotov, V.V.; et al. Long-Term Suppression of Atrial Fibrillation by Botulinum Toxin Injection Into Epicardial Fat Pads in Patients Undergoing Cardiac Surgery: One-Year Follow-Up of a Randomized Pilot Study. Circ. Arrhythm. Electrophysiol. 2015, 8, 1334–1341. [Google Scholar]
- Christensen, R.H.; Wedell-Neergaard, A.S.; Lehrskov, L.L.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Effect of Aerobic and Resistance Exercise on Cardiac Adipose Tissues: Secondary Analyses from a Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 778–787. [Google Scholar] [CrossRef]
- Ziyrek, M.; Kahraman, S.; Ozdemir, E.; Dogan, A. Metformin monotherapy significantly decreases epicardial adipose tissue thickness in newly diagnosed type 2 diabetes patients. Rev. Port. Cardiol. 2019, 38, 419–423, English. Portuguese. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Aizawa, Y.; Yuasa, S.; Fujita, S.; Ikeda, Y.; Okabe, M. The Effect of Dapagliflozin Treatment on Epicardial Adipose Tissue Volume and P-Wave Indices: An Ad-hoc Analysis of The Previous Randomized Clinical Trial. J. Atheroscler. Thromb. 2020, 27, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Mohseni, M.; Bianco, S.D.; Banga, P.K. Liraglutide causes large and rapid epicardial fat reduction. Obesity 2017, 25, 311–316. [Google Scholar] [CrossRef]
- Lima-Martínez, M.M.; Paoli, M.; Rodney, M.; Balladares, N.; Contreras, M.; D’Marco, L.; Iacobellis, G. Effect of sitagliptin on epicardial fat thickness in subjects with type 2 diabetes and obesity: A pilot study. Endocrine 2016, 51, 448–455. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Melek, B.H.; Arepalli, C.D.; Hartlage, G.R.; Chen, Z.; Kim, S.; Stillman, A.E.; Raggi, P. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: A substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J. Am. Coll. Cardiol. 2013, 61, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.; Ghannam, M.; Liang, J.; Saeed, M.; Cunnane, R.; Ghanbari, H.; Latchamsetty, R.; Crawford, T.; Batul, S.A.; Chung, E.; et al. Effect of metformin on outcomes of catheter ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2021, 32, 1232–1239. [Google Scholar] [CrossRef]
- Kato, M.; Ogano, M.; Mori, Y.; Kochi, K.; Morimoto, D.; Kito, K.; Green, F.N.; Tsukamoto, T.; Kubo, A.; Takagi, H.; et al. Exercise-based cardiac rehabilitation for patients with catheter ablation for persistent atrial fibrillation: A randomized controlled clinical trial. Eur. J. Prev. Cardiol. 2019, 26, 1931–1940. [Google Scholar] [CrossRef]
- Gaborit, B.; Venteclef, N.; Ancel, P.; Pelloux, V.; Gariboldi, V.; Leprince, P.; Amour, J.; Hatem, S.N.; Jouve, E.; Dutour, A. Clément K. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial. peri-ventricular. or peri-coronary location. Cardiovasc. Res. 2015, 108, 62–73. [Google Scholar] [CrossRef]
- Frost, L.; Vestergaard, P.; Mosekilde, L. Hyperthyroidism and risk of atrial fibrillation or flutter: A population-based study. Arch. Intern. Med. 2004, 164, 1675–1678. [Google Scholar] [CrossRef]
- Mascia, G.; Arbelo, E.; Porto, I.; Brugada, R.; Brugada, J. The arrhythmogenic right ventricular cardiomyopathy in comparison to the athletic heart. J. Cardiovasc. Electrophysiol. 2020, 31, 1836–1843. [Google Scholar] [CrossRef]
- Chamberlain, A.M.; Agarwal, S.K.; Folsom, A.R.; Duval, S.; Soliman, E.Z.; Ambrose, M.; Eberly, L.E.; Alonso, A. Smoking and incidence of atrial fibrillation: Results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm 2011, 8, 1160–1166. [Google Scholar] [CrossRef]
- Mascia, G.; Arbelo, E.; Solimene, F.; Giaccardi, M.; Brugada, R.; Brugada, J. The long-QT syndrome and exercise practice: The never-ending debate. J. Cardiovasc. Electrophysiol. 2018, 29, 489–496. [Google Scholar] [CrossRef]
- Santoro, F.; Di Biase, L.; Trivedi, C.; Burkhardt, J.D.; Paoletti Perini, A.; Sanchez, J.; Horton, R.; Mohanty, P.; Mohanty, S.; Bai, R.; et al. Impact of Uncontrolled Hypertension on Atrial Fibrillation Ablation Outcome. JACC Clin. Electrophysiol. 2015, 1, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, J.; Pallisgaard, J.; Ruwald, M.H.; Rasmussen, P.V.; Johannessen, A.; Hansen, J.; Worck, R.H.; Zörner, C.R.; Riis-Vestergaard, L.; Middelfart, C.; et al. Short- and long-term risk of atrial fibrillation recurrence after first time ablation according to body mass index: A nationwide Danish cohort study. Europace 2023, 25, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.H.; Lo, L.W.; Lin, Y.J.; Chang, S.L.; Hu, Y.F.; Hung, Y.; Chung, F.P.; Chang, T.Y.; Huang, T.C.; Yamada, S.; et al. Cigarette smoking causes a worse long-term outcome in persistent atrial fibrillation following catheter ablation. J. Cardiovasc. Electrophysiol. 2018, 29, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Guckel, D.; Isgandarova, K.; Bergau, L.; Piran, M.; El Hamriti, M.; Imnadze, G.; Braun, M.; Khalaph, M.; Fink, T.; Sciacca, V.; et al. The Effect of Diabetes Mellitus on the Recurrence of Atrial Fibrillation after Ablation. J. Clin. Med. 2021, 10, 4863. [Google Scholar] [CrossRef]
- Lu, S.; Du, X.; Yang, X.; Jia, Z.; Li, J.; Xia, S.; Chang, S.; Zuo, S.; Guo, X.; Tang, R.; et al. Physical activity and atrial tachyarrhythmia recurrence in atrial fibrillation patients after catheter ablation. Pacing Clin. Electrophysiol. PACE 2020, 43, 922–929. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, J.; Zhu, K.; Qin, F.; Liu, H.; Tao, H. The Association Between Recurrence of Atrial Fibrillation and Revascularization in Patients with Coronary Artery Disease After Catheter Ablation. Front. Cardiovasc. Med. 2021, 8, 756552. [Google Scholar] [CrossRef]
- Li, M.; Liu, T.; Luo, D.; Li, G. Systematic review and meta-analysis of chronic kidney disease as predictor of atrial fibrillation recurrence following catheter ablation. Cardiol. J. 2014, 21, 89–95. [Google Scholar] [CrossRef]



| Study | N | Males (%) | Age (y) | BMI | FU (m) | PAF (%) | HT (%) | DM (%) | CT METHOD | PVI METHOD | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nagashima et al. 2011 [25] | 40 | 77.5 | 58 (10.2) | 23 (2.6) | 10.2 | 60.0 | 37.5 | n/a | SA. HU: −50 to −200 | RF+ | Endpoint: AF BP: 2 m AADs: < 6 m |
| Tsao H. M. et al. 2011 [26] | 64 | 81.3 | 54.6 (8.5) | 25.6 (3.3) | 7.6 | 60.9 | 15.6 | 10.9 | SA. HU: −50 to −200 | RF+ | Endpoint: AF/AT BP: 3 m AADs < 8 w |
| Nakahara S. et al. 2014 [27] | 60 | 83.3 | 61.1 (10.4) | n/a | 16 | 35.0 | 78.3 | n/a | SA. HU: −50 to −200 | RF+ | Endpoint: AF/AT BP: 3 m AADs: <6 m |
| Nakatani Y. et al. 2015 [28] | 55 | 74.5 | 64 (9) | 24 (2.7) | 12 | 47.3 | 52.7 | 12.7 | SA. HU: −50 to −200 | RF+ | Endpoint: AF BP: 3 m AADs: < 3 m |
| Masuda M et al. 2015 [29] | 53 | 67.9 | 61 (11) | 24.2 (3.2) | 16 | 41.5 | 49.1 | 18.9 | SA. HU: −50 to −200 | RF+ | Endpoint: AF/AT BP: 3 m |
| Singhal R. et al. 2016 [30] | 32 | 84.4 | 58 (10) | 25 (3) | 17 | 71.9 | 56.3 | 12.5 | SA. HU: −50 to −200 | RF+ | Endpoint: AF BP: 3 m AADs < 8 w |
| Monno K. et al. 2018 [31] | 104 | 72.1 | 63 (10) | 23.9 (3.7) | 22 | 55.8 | 52.9 | 20.2 | SA. HU: −50 to −200 | RF+/CRYO | Endpoint: AF BP: 3 m |
| Kawasaki M. et al. 2019 [20] | 64 | 50 | 70.6 (9) | 23.7 (3.7) | 11 | 100 | 56.3 | 3.1 | SA. HU: −45 to −195 | RF-/CRYO | Endpoint: AF/AT BP: 3 m AADs: < 3 m |
| Nakatani Y. et al. 2020 [32] | 44 | 84.1 | 64 (10) | 25 (3) | 21 | 59.1 | 52.3 | 6.8 | SA. HU: −50 to −200 | RF+ | Endpoint: AF BP: 3 m AADs: no restriction |
| Hammache N. et al. 2021 [33] | 389 | 65.8 | 58.1 (11.1) | 27 (4.7) | 12 | 100 | 40.1 | 7.2 | SA. HU: −50 to −250 | RF- | Endpoint: AF BP: 3 m AADs: 3 m |
| Beyer C. et al. 2021 [19] | 732 | 73.2 | 57.6 (10.8) | 26.9 (4) | 31 | 88.4 | 46.2 | 4.1 | SA. HU: −45 to −195 | RF-/CRYO | Endpoint: AF BP: 3 m |
| Goldenberg G. R. et al. 2021 [34] | 130 | 68.5 | 61 (5) | 28.7 (4) | 19.5 | 67.7 | 60.8 | 26.9 | SA. HU: −45 to −195 | CRYO | Endpoint: AF/AT BP: 3 m |
| Yang M. et al. 2022 [35] | 251 | 59.0 | 62 (9) | 25 (3) | 12 | 68.9 | 54.2 | 13.9 | SA. HU: −50 to −200 | CRYO | Endpoint: AF/AT BP: 3 m AADs: < 8 w |
| Jian B. et al. 2022 [36] | 337 | 53.4 | 55.2 (12.1) | 25.3 (2.2) | 12 | n/a | n/a | 15.7 | automate. HU: −50 to −200 | RF- | Endpoint: AF/AT BP: 3 m |
| Yang M. et al. 2022 [37] | 680 | 63.8 | 62.5 (9) | 24.59 (3.1) | 12 | 0 | 50.9 | 14.7 | SA. HU: −30 to −190 | CRYO | Endpoint: AF/AT BP: 3 m AADs: < 8 w |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anagnostopoulos, I.; Kousta, M.; Kossyvakis, C.; Paraskevaidis, N.T.; Vrachatis, D.; Deftereos, S.; Giannopoulos, G. Epicardial Adipose Tissue and Atrial Fibrillation Recurrence following Catheter Ablation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 6369. https://doi.org/10.3390/jcm12196369
Anagnostopoulos I, Kousta M, Kossyvakis C, Paraskevaidis NT, Vrachatis D, Deftereos S, Giannopoulos G. Epicardial Adipose Tissue and Atrial Fibrillation Recurrence following Catheter Ablation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(19):6369. https://doi.org/10.3390/jcm12196369
Chicago/Turabian StyleAnagnostopoulos, Ioannis, Maria Kousta, Charalampos Kossyvakis, Nikolaos Taxiarchis Paraskevaidis, Dimitrios Vrachatis, Spyridon Deftereos, and Georgios Giannopoulos. 2023. "Epicardial Adipose Tissue and Atrial Fibrillation Recurrence following Catheter Ablation: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 19: 6369. https://doi.org/10.3390/jcm12196369