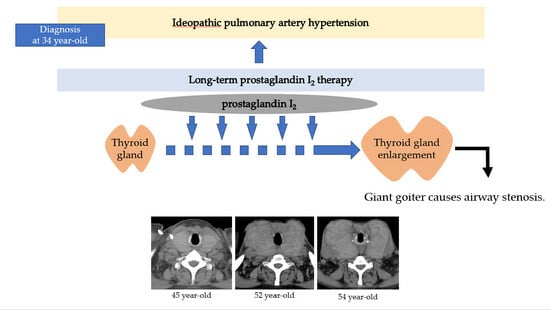
A Case of Giant Goiter Associated with Airway Stenosis Caused by Long-Term Intravenous Epoprostenol Therapy for Idiopathic Pulmonary Arterial Hypertension
Abstract
:1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Miller, D.P.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012, 142, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Akagi, S.; Nakamura, K.; Miyaji, K.; Ogawa, A.; Kusano, K.F.; Ito, H.; Matsubara, H. Marked hemodynamic improvements by high-dose epoprostenol therapy in patients with idiopathic pulmonary arterial hypertension. Circ. J. 2010, 74, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Kumamaru, H.; Satoh, T.; Miyata, H.; Ogawa, A.; Tanabe, N.; Hatano, M.; Yao, A.; Abe, K.; Tsujino, I.; et al. Effectiveness and Outcome of Pulmonary Arterial Hypertension-Specific Therapy in Japanese Patients with Pulmonary Arterial Hypertension. Circ. J. 2017, 82, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Runo, J.R.; Loyd, J.E. Primary pulmonary hypertension. Lancet 2003, 361, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Clapp, L.H.; Finney, P.; Turcato, S.; Tran, S.; Rubin, L.J.; Tinker, A. Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am. J. Respir. Cell Mol. Biol. 2002, 26, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Degering, J.; Egenlauf, B.; Harutyunova, S.; Benjamin, N.; Salkić, A.; Xanthouli, P.; Eichstaedt, C.A.; Seeger, R.; Sitbon, O.; Grünig, E. Tolerability, safety and survival in patients with severe pulmonary arterial hypertension treated with intravenous epoprostenol (Veletri®): A prospective, 6-months, open label, observational, non-interventional study. Respir. Res. 2023, 24, 18. [Google Scholar] [CrossRef]
- Galiè, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Jing, Z.C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef]
- Humbert, M.; Sitbon, O.; Simonneau, G. Treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2004, 351, 1425–1436. [Google Scholar] [CrossRef]
- Chadha, C.; Pritzker, M.; Mariash, C.N. Effect of epoprostenol on the thyroid gland: Enlargement and secretion of thyroid hormone. Endocr. Pract. 2009, 15, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Aso, K.; Nakayama, T.; Saji, T. Effect of treatment with epoprostenol and endothelin receptor antagonists on the development of thyrotoxicosis in patients with pulmonary arterial hypertension. Endocr. J. 2017, 64, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Mercé, J.; Ferrás, S.; Oltra, C.; Sanz, E.; Vendrell, J.; Simón, I.; Camprubí, M.; Bardají, A.; Ridao, C. Cardiovascular abnormalities in hyperthyroidism: A prospective Doppler echocardiographic study. Am. J. Med. 2005, 118, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, A.L.; Sheahan, P.; Timon, C. Management of life-threatening airway obstruction caused by benign thyroid disease. J. Laryngol. Otol. 2006, 120, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhou, H.; Ren, G.; Wang, Y.; Sui, Y. A new treatment strategy for airway obstruction induced by a giant benign goiter: A case report. Exp. Ther. Med. 2023, 26, 376. [Google Scholar] [CrossRef] [PubMed]
- Raftos, J.R.; Ethell, A.T. Goitre causing acute respiratory arrest. Aust. N. Z. J. Surg. 1996, 66, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Abughazaleh, S.; Safdar, Z. Goiter in a Patient with Pulmonary Arterial Hypertension Treated with Epoprostenol. Case Rep. Pulmonol. 2020, 2020, 1617253. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, K.; Okada, Y.; Nakayamada, S.; Kubo, S.; Kurozumi, A.; Narisawa, M.; Tanaka, Y. Comprehensive immunophenotypic analysis reveals the pathological involvement of Th17 cells in Graves’ disease. Sci. Rep. 2022, 12, 16880. [Google Scholar] [CrossRef]
- Boswell, M.G.; Zhou, W.; Newcomb, D.C.; Peebles, R.S., Jr. PGI2 as a regulator of CD4+ subset differentiation and function. Prostaglandins Other Lipid Mediat. 2011, 96, 21–26. [Google Scholar] [CrossRef]
- Kasai, K.; Hiraiwa, M.; Suzuki, Y.; Banba, N.; Emoto, T.; Nakamura, T.; Shimoda, S.I. Prostacyclin stimulation of adenylate cyclase activity in human thyroid membranes. Horm. Metab. Res. 1986, 18, 625–629. [Google Scholar] [CrossRef]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Bernet, V.J.; Carty, S.E.; Davies, T.F.; Ganly, I.; Inabnet, W.B., 3rd; Shaha, A.R. American Thyroid Association statement on optimal surgical management of goiter. Thyroid 2014, 24, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ríos, A.; Rodríguez, J.M.; Canteras, M.; Galindo, P.J.; Tebar, F.J.; Parrilla, P. Surgical management of multinodular goiter with compression symptoms. Arch. Surg. 2005, 140, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sako, H.; Nakane, Y.; Okugawa, K.; Nakano, K.; Yamano, T. A case of graves’disease with an enormous goiter. Nihon Rinsho Geka Gakkai Zasshi (J. Jpn. Surg. Assoc.) 2004, 65, 2848–2852. [Google Scholar] [CrossRef]
- Price, L.C.; Martinez, G.; Brame, A.; Pickworth, T.; Samaranayake, C.; Alexander, D.; Garfield, B.; Aw, T.C.; McCabe, C.; Mukherjee, B.; et al. Perioperative management of patients with pulmonary hypertension undergoing non-cardiothoracic, non-obstetric surgery: A systematic review and expert consensus statement. Br. J. Anaesth. 2021, 126, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, S.A.; Taboada, D.; Martinez, G. Pulmonary hypertension and its management in patients undergoing non-cardiac surgery. Anaesthesia 2015, 70, 56–70. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiura, K.; Nakazato, K.; Yokokawa, T.; Suzuki, Y.; Kurosawa, Y.; Wada, K.; Shimizu, T.; Oikawa, M.; Kobayashi, A.; Sugimoto, K.; et al. A Case of Giant Goiter Associated with Airway Stenosis Caused by Long-Term Intravenous Epoprostenol Therapy for Idiopathic Pulmonary Arterial Hypertension. J. Clin. Med. 2023, 12, 6359. https://doi.org/10.3390/jcm12196359
Nishiura K, Nakazato K, Yokokawa T, Suzuki Y, Kurosawa Y, Wada K, Shimizu T, Oikawa M, Kobayashi A, Sugimoto K, et al. A Case of Giant Goiter Associated with Airway Stenosis Caused by Long-Term Intravenous Epoprostenol Therapy for Idiopathic Pulmonary Arterial Hypertension. Journal of Clinical Medicine. 2023; 12(19):6359. https://doi.org/10.3390/jcm12196359
Chicago/Turabian StyleNishiura, Kazuto, Kazuhiko Nakazato, Tetsuro Yokokawa, Yoshinori Suzuki, Yuta Kurosawa, Kento Wada, Takeshi Shimizu, Masayoshi Oikawa, Atsushi Kobayashi, Koichi Sugimoto, and et al. 2023. "A Case of Giant Goiter Associated with Airway Stenosis Caused by Long-Term Intravenous Epoprostenol Therapy for Idiopathic Pulmonary Arterial Hypertension" Journal of Clinical Medicine 12, no. 19: 6359. https://doi.org/10.3390/jcm12196359