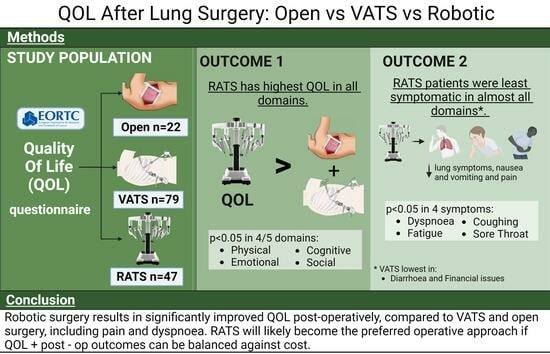
Comparison of Quality of Life after Robotic, Video-Assisted, and Open Surgery for Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pre- and Post-RATS
2.2. RATS, VATS, and Thoracotomy
2.3. Statistical Analysis
3. Results
3.1. Pre- and Post-RATS
Quality of Life Results
3.2. RATS vs. VATS vs. Thoracotomy
Quality of Life Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, A.; Gallesio, J.M.A.; Sewell, D.B.; Carr, R.; Molena, D. Outcomes of robotic esophagectomy. J. Thorac. Dis. 2021, 13, 6163–6168. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, R.S.; Patton, B.D.; Wasserman, G.A.; Karp, J.; Cohen, S.; Inra, M.L.; Scheinerman, S.J. Robotic-assisted tracheobronchoplasty: Quality of life and pulmonary function assessment on intermediate follow-up. J. Thorac. Cardiovasc. Surg. 2021, 164, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Suda, T. Transition from video-assisted thoracic surgery to robotic pulmonary surgery. J. Vis. Surg. 2017, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G.; Galetta, D.; Maisonneuve, P.; Melfi, F.; Schmid, R.A.; Borri, A.; Vannucci, F.; Spaggiari, L. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 140, 19–25. [Google Scholar] [CrossRef]
- Flores, R.M.; Alam, N. Video-Assisted Thoracic Surgery Lobectomy (VATS), Open Thoracotomy, and the Robot for Lung Cancer. Ann. Thorac. Surg. 2008, 85, S710–S715. [Google Scholar] [CrossRef]
- Ng, C.S.H.; MacDonald, J.K.; Gilbert, S.; Khan, A.Z.; Kim, Y.T.; Louie, B.E.; Marshall, M.B.; Santos, R.S.; Scarci, M.; Shargal, Y.; et al. Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systemic Review and Meta-Analysis. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2019, 14, 90–116. [Google Scholar] [CrossRef]
- Louie, B.E.; Farivar, A.S.; Aye, R.W.; Vallières, E. Early Experience with Robotic Lung Resection Results in Similar Operative Outcomes and Morbidity when Compared with Matched Video-Assisted Thoracoscopic Surgery Cases. Ann. Thorac. Surg. 2012, 93, 1598–1605. [Google Scholar] [CrossRef]
- Yang, H.X.; Woo, K.M.; Sima, C.S.; Bains, M.S.; Adusumilli, P.S.; Huang, J.; Finley, D.J.; Rizk, N.P.; Rusch, V.W.; Jones, D.R.; et al. Long-Term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Non-Small Cell Lung Cancer: Comparison of Robotic, Video Assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg. 2017, 265, 431. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bryant, A.S.; Skylizard, L.; Minnich, D.J. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J. Thorac. Cardiovasc. Surg. 2011, 142, 740–746. [Google Scholar] [CrossRef]
- Veronesi, G.; Abbas, A.E.-S.; Muriana, P.; Lembo, R.; Bottoni, E.; Perroni, G.; Testori, A.; Dieci, E.; Bakhos, C.T.; Car, S.; et al. Perioperative Outcome of Robotic Approach Versus Manual Videothoracoscopic Major Resection in Patients Affected by Early Lung Cancer: Results of a Randomized Multicentric Study (ROMAN Study). Front. Oncol. 2021, 11, 726408. [Google Scholar] [CrossRef]
- Fang, W.T.; Chen, T.B.; Luo, J.Z.; Ji, C.Y.; Yao, F. Minimally invasive surgery for centrally located lung cancers. Zhonghua Wai Ke Za Zhi 2020, 58, 57–60. [Google Scholar] [PubMed]
- Lim, E.; Batchelor, T.J.; Dunning, J.; Shackcloth, M.; Anikin, V.; Naidu, B.; Belcher, E.; Loubani, M.; Zamvar, V.; Harris, R.A.; et al. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid. 2022, 1. [Google Scholar] [CrossRef]
- Balduyck, B.; Hendriks, J.; Lauwers, P.; Van Schil, P. Quality of life evolution after lung cancer surgery: A prospective study in 100 patients. Lung Cancer 2007, 56, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Handy, J.R., Jr.; Asaph, J.W.; Douville, E.C.; Ott, G.Y.; Grunkemeier, G.L.; Wu, Y. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur. J. Cardiothorac. Surg. 2010, 37, 451–455. [Google Scholar] [CrossRef]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Bergman, B.; Aaronson, N.; Ahmedzai, S.; Kaasa, S.; Sullivan, M.; EORTC Study Group on Quality of Life. The EORTC QLQ-LC13: A modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. Eur. J. Cancer 1994, 30, 635–642. [Google Scholar] [CrossRef]
- Marzorati, C.; Mazzocco, K.; Monzani, D.; Pavan, F.; Casiraghi, M.; Spaggiari, L.; Monturano, M.; Pravettoni, G. One-Year Quality of Life Trends in Early-Stage Lung Cancer Patients After Lobectomy. Front. Psychol. 2020, 11, 534428. [Google Scholar] [CrossRef]
- Pompili, C. Quality of life after lung resection for lung cancer. J. Thorac. Dis. 2015, 7 (Suppl. S2), S138–S144. [Google Scholar] [CrossRef]
- Singer, E.S.; Kneuertz, P.J.; Nishimura, J.; D’souza, D.M.; Diefenderfer, E.; Moffatt-Bruce, S.D.; Merritt, R.E. Effect of operative approach on quality of life following anatomic lung cancer resection. J. Thorac. Dis. 2020, 12, 6913–6919. [Google Scholar] [CrossRef]
- Cao, C.; Manganas, C.; Ang, S.C.; Yan, T.D. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann. Cardiothorac. Surg. 2012, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, V.; Kahn, D.; Matte, P.; Pieters, T.; Noirhomme, P.; Poncelet, A.; Steyaert, A. Robotic-Assisted Lobectomy Favors Early Lung Recovery versus Limited Thoracotomy. Thorac. Cardiovasc. Surg. 2021, 69, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, V.; Nezhad, Z.M.; Kahn, D.; Steyaert, A.; Poncelet, A.; Pieters, T.; Noirhomme, P. Pain, Quality of Life, and Clinical Outcomes after Robotic Lobectomy. Thorac. Cardiovasc. Surg. 2016, 65, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Zhao, L.; Grenda, T.R.; Kathawate, R.G.; Biesterveld, B.E.; Bhatti, U.F.; Carrott, P.W.; Lagisetty, K.H.; Chang, A.C.; Lynch, W.; et al. Higher Long-term Quality of Life Metrics After Video-Assisted Thoracoscopic Surgery Lobectomy Com-pared With Robotic-Assisted Lobectomy. Ann. Thorac. Surg. 2020, 113, 1591–1597. [Google Scholar] [CrossRef]
- Worrell, S.G.; Dedhia, P.; Gilbert, C.; James, C.; Chang, A.C.; Lin, J.; Reddy, R.M. The cost and quality of life outcomes in developing a robotic lobectomy program. J. Robot. Surg. 2019, 13, 239–243. [Google Scholar] [CrossRef]
- Koller, M.; Hjermstad, M.; Tomaszewski, K.; Tomaszewska, I.; Hornslien, K.; Harle, A.; Arraras, J.; Morag, O.; Pompili, C.; Ioannidis, G.; et al. An international study to revise the EORTC questionnaire for assessing quality of life in lung cancer patients. Ann. Oncol. 2017, 28, 2874–2881. [Google Scholar] [CrossRef]
- Pompili, C.; Koller, M.; Velikova, G. Choosing the right survey: The lung cancer surgery. J. Thorac. Dis. 2020, 12, 6892–6901. [Google Scholar] [CrossRef]






| (N = 47) | ||
|---|---|---|
| Age (Median) | 69.8 ± 9.1 | |
| Gender | Male | 15 (34.0%) |
| Female | 32 (68.1%) | |
| PS ECOG | 0 | 12 (25.5%) |
| 1 | 23 (48.9%) | |
| 2 | 12 (25.5%) | |
| Smoking status | Non smoker | 10 (25.5%) |
| Ex−smoker | 29 (61.7%) | |
| Smoker | 8 (17.0%) | |
| Comorbidities | Pulmonary | 12(25.5%) |
| Cardiac | 26 (55.3%) | |
| Previous cancer | 11 (23.4%) | |
| Of which is primary lung cancer | 2 (4.3%) | |
| Nil | 5 (10.6%) | |
| Procedure | Lobectomy | 33 (70.2%) |
| Bi lobectomy | 0 | |
| Segmentectomy | 10 (21.3%) | |
| Wedge resection | 4 (8.5%) | |
| Number of Ports | 4 | 47 (100%) |
| Operating Time: | ||
| Mean (±SD), mins | 110.8 (±38.8) | − |
| Median (IQR), mins | 105 (41) | − |
| Pre-Operative Staging | IA1 | 4 (8.5%) |
| IA2 | 15 (31.9%) | |
| IA3 | 12 (25.5%) | |
| IB | 1 (2.1%) | |
| IIA | 2 (4.3%) | |
| IIB | 2 (4.3%) | |
| IIIA | 2 (4.3%) | |
| IVB 1 | 1 (2.1%) | |
| Secondary Lung Metastases | 7 (14.9%) | |
| No Pre-op Staging | 1 (2.1%) | |
| Post-Operative Staging | IA1 | 2 (4.3%) |
| IA2 | 13 (27.7%) | |
| IA3 | 10 (21.3%) | |
| IB | 6 (12.8%) | |
| IIA | 1 (2.1%) | |
| IIB | 5 (10.6%) | |
| IIIA | 2 (4.3%) | |
| IIIB | 1 (2.1%) | |
| No Staging (Secondary Metastasis) | 7 (14.9%) | |
| Post-Operative Histology | Adenocarcinoma | 33 (70.2%) |
| Squamous Cell Carcinoma | 3 (6.4%) | |
| Metastasis | 7 (14.9%) | |
| Carcinoid | 4 (8.5%) | |
| Complications | Inpatient complications | 17 (36.2%) |
| Of which: | ||
| COVID | 2 (4.3%) | |
| Prolonged AL (>7 days) | 5 (10.6%) | |
| AF | 3 (6.4%) | |
| Atelectasis/sputum plug/bronchoscopy | 3 (6.4%) | |
| Hospital Acquired Pneumonia (HAP) | 4 (8.5%) | |
| Pleural effusion/empyema | 2 (4.3%) | |
| Pneumothorax—new drain insertion | 1 (2.1%) | |
| Clavien–Dindo | 0 | 30 (63.8%) |
| 1 | 9 (19.2%) | |
| 2 | 5 (10.6%) | |
| 3 | 0 | |
| 3a | 2 (4.3%) | |
| 4a | 1 (2.1%) | |
| Thoracotomy (N = 22) | VATS (N = 79) | RATS (N = 47) | p-Value | ||
|---|---|---|---|---|---|
| Age | 68.2 ± 8.8 | 75.6 ± 9.6 | 69.8 ± 9.1 | <0.001 | |
| Gender | Male | 11 (50.0%) | 33 (41.8%) | 15 (31.9%) | 0.32 |
| Female | 11 (50.0%) | 46 (58.2%) | 32 (68.1%) | ||
| PS ECOG | 0 | 6 (27.3%) | 15 (19.0%) | 12 (25.5%) | 0.86 |
| 1 | 11 (50.0%) | 45 (57.0%) | 23 (48.9%) | ||
| 2 | 5 (22.7%) | 19 (24.0%) | 12 (25.5%) | ||
| Smoking Status | Non-Smoker | 2 (9.1%) | 9 (11.4%) | 12 (25.5%) | 0.24 |
| Ex-smoker | 16 (72.7%) | 58 (73.4%) | 27 (57.5%) | ||
| Smoker | 4 (18.2%) | 12 (15.2%) | 8 (17.0%) | ||
| Co-morbidities | Pulmonary | 7 (31.8%) | 28 (35.4%) | 12 (25.5%) | 0.51 |
| Cardiac | 11 (50.0%) | 54 (68.4%) | 26 (55.3%) | 0.17 | |
| Renal | − | 4 (5.1%) | 1 (2.1%) | 0.57 | |
| Previous cancer | 6 (27.3%) | 25 (31.6%) | 11 (23.4%) | 0.61 | |
| Previous primary lung cancer | 2 (9.1%) | 2 (2.5%) | 2 (4.3%) | 0.29 | |
| Pre-Operative Staging | IA1 | 0 (0%) | 12 (15.2%) | 4 (8.5%) | |
| IA2 | 2 (9.1%) | 27 (34.2%) | 15 (31.9%) | ||
| IA3 | 1 (4.5%) | 8 (10.1%) | 12 (25.5%) | ||
| IB | 2 (9.1%) | 14 (17.7%) | 1 (2.1%) | ||
| IIA | 1 (4.5%) | 4 (5.1%) | 2 (4.3%) | ||
| IIB | 6 (27.3%) | 8 (10.1%) | 2 (4.3%) | ||
| IIIA | 4 (18.2%) | 0 | 2 (4.3%) | ||
| IIIB | 2 (9.1%) | 1 (1.3%) | 0 | ||
| IIIC | 1 (4.5%) | 0 | 0 | ||
| IV | 3 (13.6%) | 0 | 1 (2.1%) | ||
| Secondary Metastasis | 3 (3.8%) | 7 (14.9%) | |||
| No Staging (No Pre-op Staging) | 2 (2.5%) | 1 (2.1%) | |||
| Procedure | Lobectomy | 15 (68.2%) | 50 (63.3%) | 33 (70.2%) | |
| Pneumonectomy | 2 (9.1%) | 0 | 0 | ||
| Segmentectomy | 2 (9.1%) | 17 (21.5%) | 10 (21.3%) | ||
| Wedge resection | 3 (13.6%) | 12 (15.2%) | 4 (8.5%) | ||
| Number of Ports | 3 | − | 79 (100%) | 0 | |
| 4 | − | 0 | 47 (100%) | ||
| Operating Time: | |||||
| Mean (+/−SD), mins | 143.2 (±38.4) | 116.1 (±32.2) | 110.8 (±38.8) | ||
| Median (IQR), mins | 142 (60) | 120 (51.25) | 105 (41) | ||
| Conversion | Yes | N/A | 6 (7.6%) | 0 | |
| No | N/A | 73 (92.4%) | 47 (100%) | ||
| Final Staging | IA1 | 0 | 4 (5.1%) | 2 (4.3%) | |
| IA2 | 0 | 18 (22.8%) | 13 (27.7%) | ||
| IA3 | 0 | 9 (11.4%) | 10 (21.3%) | ||
| IB | 3 (13.6%) | 17 (21.5%) | 6 (12.8%) | ||
| IIA | 2 (9.1%) | 5 (6.3%) | 1 (2.1%) | ||
| IIB | 5 (22.7%) | 11 (13.9%) | 5 (10.6%) | ||
| IIIA | 5 (22.7%) | 6 (7.6%) | 2 (4.3%) | ||
| IIIB | 2 (9.1%) | 1 (1.3%) | 1 (2.1%) | ||
| 0 (no staging) | 1 (4.5) | 0 | 0 | ||
| Secondary Metastasis | 4 (18.2%) | 5 (6.3%) | 7 (14.9%) | ||
| Benign Disease | 0 | 1 (1.3%) | 0 | ||
| Carcinoid | 0 | 1 (1.3%) | 0 | ||
| TNM Staging Not Applicable 1 | 0 | 1 (1.3%) | 0 | ||
| Complications | In-hospital complications | 6 (27.3%) | 21 (26.6%) | 17 (36.2%) | 0.50 |
| COVID | 0 | 0 | 2 (4.3%) | 0.22 | |
| Prolonged air leak (>7 days) | 2 (9.1%) | 3 (3.8%) | 5 (10.6%) | 0.23 | |
| AF | 0 | 9 (11.4%) | 3 (6.4%) | 0.24 | |
| Airway complications | 1 (4.5%) | 4 (5.1%) | 3 (6.4%) | >0.99 | |
| HAP | 4 (18.2%) | 12 (15.2%) | 4 (8.5%) | 0.48 | |
| Pleural effusion/empyema | 0 | 1 (1.3%) | 2 (4.3%) | 0.73 | |
| Surgical emphysema | 0 | 2 (2.5%) | 0 | 0.66 | |
| Pneumothorax—new drain insertion | 0 | 1 (1.3%) | 1 (2.1%) | >0.99 | |
| Clavien–Dindo | 0 | 16 (72.7%) | 58 (73.4%) | 30 (63.8%) | 0.083 |
| 1 | 2 (9.1%) | 3 (3.8%) | 9 (19.1%) | ||
| 2 | 3 (13.6%) | 15 (19%) | 5 (10.6%) | ||
| 3 | 1 (4.5%) | 2 (2.5%) | 0 | ||
| 3a | 0 | 1 (1.3%) | 2 (4.3%) | ||
| 4a | 0 | 0 | 1 (2.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asemota, N.; Maraschi, A.; Lampridis, S.; Pilling, J.; King, J.; Le Reun, C.; Bille, A. Comparison of Quality of Life after Robotic, Video-Assisted, and Open Surgery for Lung Cancer. J. Clin. Med. 2023, 12, 6230. https://doi.org/10.3390/jcm12196230
Asemota N, Maraschi A, Lampridis S, Pilling J, King J, Le Reun C, Bille A. Comparison of Quality of Life after Robotic, Video-Assisted, and Open Surgery for Lung Cancer. Journal of Clinical Medicine. 2023; 12(19):6230. https://doi.org/10.3390/jcm12196230
Chicago/Turabian StyleAsemota, Nicole, Alessandro Maraschi, Savvas Lampridis, John Pilling, Juliet King, Corinne Le Reun, and Andrea Bille. 2023. "Comparison of Quality of Life after Robotic, Video-Assisted, and Open Surgery for Lung Cancer" Journal of Clinical Medicine 12, no. 19: 6230. https://doi.org/10.3390/jcm12196230