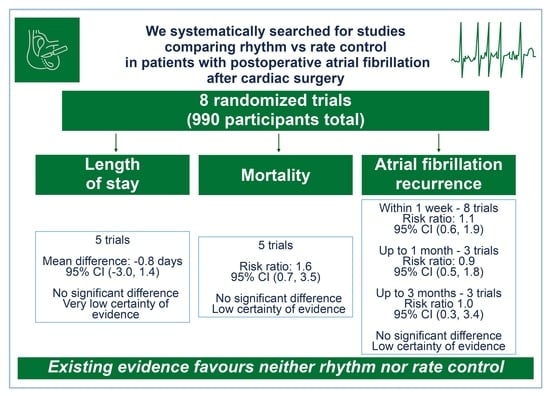
Rhythm vs. Rate Control in Patients with Postoperative Atrial Fibrillation after Cardiac Surgery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Methods
2.3. Selection of Studies
2.4. Data Extraction
2.5. Risk of Bias
2.6. Statistical Analysis
2.7. Quality Assessment
3. Results
3.1. Selection of Included Studies
3.2. Risk of Bias Assessment
4. Outcomes
4.1. Data from Randomized Trials
4.2. Data from Observational Studies
5. Discussion
6. Strengths and Weaknesses
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| Atrial fibrillation | (AF) |
| Canadian Cardiovascular Society | (CCS) |
| Coronary artery bypass graft | (CABG) |
| Direct current cardioversion | (DCCV) |
| European Society of Cardiology | (ESC) |
| European Association for Cardio-Thoracic Surgery | (EACTS) |
| Given intravenously | (IV) |
| GRADE | (Grading of Recommendations Assessment, Development and Evaluation) |
| Postoperative atrial fibrillation | (POAF) |
| Preferred Reporting Items for Systematic Reviews and Meta-Analyses | (PRISMA) |
| Taken orally | (PO) |
References
- Writing Group Members; Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; et al. Heart Disease and Stroke Statistics—2012 Update. Circulation 2012, 125, e2–e220. [Google Scholar]
- Yusuf, S.; Reddy, S.; Ounpuu, S.; Anand, S. Global burden of cardiovascular diseases: Part II: Variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation 2001, 104, 2855–2864. [Google Scholar] [CrossRef] [Green Version]
- Baghai, M.; Wendler, O.; Grant, S.W.; Goodwin, A.T.; Trivedi, U.; Kendall, S.; Jenkins, D.P. Aortic valve surgery in the UK, trends in activity and outcomes from a 15-year complete national series. Eur. J. Cardio-Thorac. Surg. 2021, 60, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Bessissow, A.; Khan, J.; Devereaux, P.J.; Alvarez-Garcia, J.; Alonso-Coello, P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: An overview. J. Thromb. Haemost. 2015, 13 (Suppl. S1), S304–S312. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, R.; Sanjanwala, R.; Le, M.-L.; Yamashita, M.H.; Arora, R.C. Postoperative Atrial Fibrillation after Cardiac Surgery: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2021, 111, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.K.; Meyre, P.B.; Heo, R.; Devereaux, P.J.; Birchenough, L.; Whitlock, R.; McIntyre, W.F.; Chen, Y.C.P.; Ali, M.Z.; Biancari, F.; et al. Short-term and Long-term Risk of Stroke in Patients with Perioperative Atrial Fibrillation after Cardiac Surgery: Systematic Review and Meta-analysis. CJC Open 2022, 4, 85–96. [Google Scholar] [CrossRef]
- Goyal, P.; Kim, M.; Krishnan, U.; Mccullough, S.A.; Cheung, J.W.; Kim, L.K.; Pandey, A.; A Borlaug, B.; Horn, E.M.; Safford, M.M.; et al. Post-operative atrial fibrillation and risk of heart failure hospitalization. Eur. Heart J. 2022, 43, 2971–2980. [Google Scholar] [CrossRef]
- LaPar, D.J.; Speir, A.M.; Crosby, I.K.; Fonner, E., Jr.; Brown, M.; Rich, J.B.; Quader, M.; Kern, J.A.; Kron, I.L.; Ailawadi, G. Postoperative Atrial Fibrillation Significantly Increases Mortality, Hospital Readmission, and Hospital Costs. Ann. Thorac. Surg. 2014, 98, 527–533. [Google Scholar] [CrossRef]
- Macle, L.; Cairns, J.; Leblanc, K.; Tsang, T.; Skanes, A.; Cox, J.L.; Healey, J.S.; Bell, A.; Pilote, L.; Andrade, J.G.; et al. 2016 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can. J. Cardiol. 2016, 32, 1170–1185. [Google Scholar] [CrossRef] [Green Version]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Brieger, D.; Amerena, J.; Attia, J.; Bajorek, B.; Chan, K.H.; Connell, C.; Freedman, B.; Ferguson, C.; Hall, T.; Haqqani, H.; et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ. 2018, 27, 1209–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa-Uva, M.; Head, S.J.; Milojevic, M.; Collet, J.-P.; Landoni, G.; Castella, M.; Dunning, J.; Gudbjartsson, T.; Linker, N.J.; Sandoval, E.; et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur. J. Cardiothorac. Surg. 2018, 53, 5–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 14898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Clarity Review Group, McMaster University. Tool to Assess Risk of Bias in Cohort Studies. Available online: http://help.magicapp.org/knowledgebase/articles/327941-tool-to-assess-risk-of-bias-in-cohort-studies (accessed on 10 May 2023).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Hjelms, E. Procainamide conversion of acute atrial fibrillation after open-heart surgery compared with digoxin treatment. Scand. J. Thorac. Cardiovasc. Surg. 1992, 26, 193–196. [Google Scholar] [CrossRef]
- Kamali, A.; Sanatkar, A.; Sharifi, M.; Moshir, E. Evaluation of amiodarone versus metoprolol in treating atrial fibrillation after coronary artery bypass grafting. Interv. Med. Appl. Sci. 2017, 9, 51–55. [Google Scholar] [CrossRef]
- Lee, J.K.; Klein, G.J.; Krahn, A.D.; Yee, R.; Zarnke, K.; Simpson, C.; Skanes, A.; Spindler, B. Rate-control versus conversion strategy in postoperative atrial fibrillation: A prospective, randomized pilot study. Am. Heart J. 2000, 140, 871–877. [Google Scholar] [CrossRef]
- Soucier, R.; Silverman, D.; Abordo, M.; Jaagosild, P.; Abiose, A.; Madhusoodanan, K.P.; Therrien, M.; Lippman, N.; Dalamagas, H.; Berns, E. Propafenone versus ibutilide for post operative atrial fibrillation following cardiac surgery: Neither strategy improves outcomes compared to rate control alone (the PIPAF study). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2003, 9, PI19–PI23. [Google Scholar]
- Wafa, S.S.; Ward, D.E.; Parker, D.J.; Camm, A.J. Efficacy of flecainide acetate for atrial arrhythmias following coronary artery bypass grafting. Am. J. Cardiol. 1989, 63, 1058–1064. [Google Scholar] [CrossRef]
- Yilmaz, A.T.; Demírkiliç, U.; Arslan, M.; Kurulay, E.; Ozal, E.; Tatar, H.; Öztürk, Y. Long-term prevention of atrial fibrillation after coronary artery bypass surgery: Comparison of quinidine, verapamil, and amiodarone in maintaining sinus rhythm. J. Card. Surg. 1996, 11, 61–64. [Google Scholar] [CrossRef]
- Gillinov, A.M.; Bagiella, E.; Moskowitz, A.J.; Raiten, J.M.; Groh, M.A.; Bowdish, M.E.; Ailawadi, G.; Kirkwood, K.A.; Perrault, L.P.; Parides, M.K.; et al. Rate Control versus Rhythm Control for Atrial Fibrillation after Cardiac Surgery. N. Engl. J. Med. 2016, 374, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Karaçağlar, E.; Atar, İ.; Özbiçer, S.; Sezgin, A.; Özçobanoğlu, S.; Yazici, A.C.; Özin, B.; Müderrisoğlu, H. Amiodarone Versus Direct Current Cardioversion in Treatment of Atrial Fibrillation after Cardiac Surgery. Turk. J. Clin. Lab. 2019, 10, 26–32. Available online: https://dergipark.org.tr/en/pub/tjcl/issue/44073/519537#article_cite (accessed on 8 December 2021). [CrossRef] [Green Version]
- Kowey, P.R.; Stebbins, D.; Igidbashian, L.; Goldman, S.M.; Sutter, F.P.; Rials, S.J.; Marinchak, R.A. Clinical outcome of patients who develop PAF after CABG surgery. Pacing Clin. Electrophysiol. 2001, 24, 191–193. [Google Scholar] [CrossRef]
- Abbas, S.; Gul, S.; Dhahri, A.; Iqbal, M.; Khan, T.; Khan, S. Amiodarone vs digoxin in the treatment of atrial fibrillation in postoperative rheumatic cardiac valvular patients. J. Pak. Med. Assoc. 2016, 66, 1098–1101. [Google Scholar]
- Bruggmann, C.; Astaneh, M.; Lu, H.; Tozzi, P.; Ltaief, Z.; Voirol, P.; Sadeghipour, F. Management of Atrial Fibrillation Following Cardiac Surgery: Observational Study and Development of a Standardized Protocol. Ann. Pharmacother. 2021, 55, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, G.; Cemin, C.; Russo, T.E.; Pellegrini, A.; Terrasi, F.; Ferrario, G. Post-discharge recurrences of new-onset atrial fibrillation following cardiac surgery: Impact of low-dose amiodarone and beta-blocker prophylaxis. Ital. Heart J. 2000, 1, 691–697. [Google Scholar] [PubMed]
- List of Abstracts: Suppl. 1 to Vol. 12 (May 20, 2011). Interact. Cardiovasc. Thorac. Surg. 2011, 12 (Suppl. S1), iv–xxiii. [CrossRef]
- Guaragna, J.C.; Martins, V.; Brunini, T.M.; Linhatti, J.L.; Brauner, F.B.; Pires, R.C.; Bodanese, L.C. Use of high-dose oral amiodarone for the reversion of atrial fibrillation during the postoperative period of cardiac surgery. Arq. Bras. Cardiol. 1997, 69, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.; Shpigel, A.; Wasser, T.; Sabo, M.; Feldman, B. Morbidity of Post-Coronary Artery Bypass Surgery Patients with Atrial Fibrillation Treated with Rate Control versus Sinus-Restoring Therapy. Heart Drug 2001, 1, 192–196. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Ashraf Ali, A.; Mohamed, A.; el Sherif, L.; Abdelsamed, M.; Kamal, M.; Sayed, M.K.; Mohamed, N.A.; Osman, A.A.; Shaheen, S.M.; et al. Rhythm Versus Rate Control for Atrial Fibrillation: A Meta-analysis of Randomized Controlled Trials. Biomed. Pharmacol. J. 2018, 11, 609–620. [Google Scholar] [CrossRef]
- ICHOM Our Mission. Available online: https://www.ichom.org/mission/ (accessed on 8 March 2022).
- Seligman, W.H.; Das-Gupta, Z.; Jobi-Odeneye, A.O.; Arbelo, E.; Banerjee, A.; Bollmann, A.; Caffrey-Armstrong, B.; A Cehic, D.; Corbalan, R.; Collins, M.; et al. Development of an international standard set of outcome measures for patients with atrial fibrillation: A report of the International Consortium for Health Outcomes Measurement (ICHOM) atrial fibrillation working group. Eur. Heart J. 2020, 41, 1132–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovaris, G.; Ciconte, G.; Schiavone, M.; Mitacchione, G.; Gasperetti, A.; Piazzi, E.; Negro, G.; Montemerlo, E.; Rondine, R.; Pozzi, M.; et al. Second-generation laser balloon ablation for the treatment of atrial fibrillation assessed by continuous rhythm monitoring: The LIGHT-AF study. Europace 2021, 23, 1380–1390. [Google Scholar] [CrossRef] [PubMed]




| Study ID | N | Surgery Type | Rhythm Control | Rate Control | Follow-Up Duration | Outcomes Reported |
|---|---|---|---|---|---|---|
| Demirkilic 1996 [24] | 120 | Isolated CABG: 120/120 (100%) | Choice between: -Quinidine PO 550 mg/day -Amiodarone PO 600 mg/day for 7 days then 200 mg/day | Verapamil PO 240 mg/day | 1 week | AF recurrence (within 1 week, up to 1 month & up to 3 months) |
| Gillinov 2016 [25] | 523 | Isolated CABG: 212/523 (40.5%) CABG + valve repair: 17/523 (3.3%) CABG + valve replacement: 86/523 (16.4%) Non-CABG: 208/523 (39.8%) | Amiodarone 3 g PO load then 200 mg per day Both arms received rate control for HR < 100 and got DCCV if AF was persistent beyond 24–48 h | Beta blocker and/or calcium channel blocker and/or Digoxin Both arms received rate control for HR < 100 and got DCCV if AF was persistent beyond 24–48 h | 60 days | Length of stay AF recurrence (within 1 week, up to 1 month & up to 3 months) Mortality Stroke |
| Hjelms 1992 [19] | 30 | Isolated CABG: 25/30 (83.3%) Non-CABG: 5/30 (16.7%) | IV Procainamide, then PO Procainamide for 1 week | Choice between: -IV Digoxin -PO Digoxin maintenance dose 0.1–0.3 mg | 1 week | Length of stay AF recurrence (within 1 week) Mortality |
| Kamali 2017 [20] | 146 | Isolated CABG: 146/146 (100%) | Amiodarone PO or IV 300 mg followed by 1–3 mg/kg every 6 h and 0.5 mg/kg 18 h later | Beta blocker IV 1–3 mg/kg/h for 24 h | 24 h | Length of stay AF Recurrence (within 1 week) Mortality |
| Karacaglar 2019 [26] | 50 | Isolated CABG: 43/50 (86%)CABG + valve surgery: 7/50 (14%) | IV amiodarone DCCV if in AF at 24 h then PO amiodarone for 28 days | Beta blocker, calcium channel blocker or Digoxin DCCV if in AF at 24 h | 30 days | Length of stay AF Recurrence (within 1 week, up to 1 month) Bleeding Mortality Stroke |
| Lee 2000 [21] | 50 | Isolated CABG: 34/50 (68%)CABG + valve surgery: 7/50 (14%) Non-CABG: 9/50 (18%) | Choice between: -Sotalol PO 120–360 mg/day -Propafenone PO 300–900 mg/day -Procainamide IV 500–1000 mg followed by a continuous infusion of 1 to 4 mg/h or 2 to 3 g/day in divided oral doses. -Amiodarone IV 200 mg/day after a loading dose of 1200 to 1600 mg for 4 to 5 days DCCV if in AF at 48 h | Beta blocker, calcium channel blocker or Digoxin | Rhythm: 48 h Rate: Until HR ≤ 110 BPM or 110–120 BPM with no heart failure | Length of stay AF recurrence (within 1 week, up to 1 month & up to 3 months) Mortality |
| Soucier 2003 [22] | 42 | Isolated CABG: 34/42 (81%)CABG + valve surgery: 6/42 (14.3%) Non-CABG: 2/42 (4.8%) | Choice between: -IV Ibutilide -Propafenone | Physician choicebeta blocker encouraged | 1 week | AF recurrence (within 1 week) Stroke |
| Wafa 1989 [23] | 29 | Isolated CABG: 29/29 (100%) | Flecainide IV for up to 24 h | IV Digoxin +/− Verapamil | 24 h | Length of stay AF recurrence (within 1 week) |
| Group | N Studies (References) | Total Patients | Mean Length of Stay in Days+/− Standard Deviation | MeanDifference (95%CI) | p-Value | I2n | Quality of Evidence Reason for Judgement (Supplementary Files S7, S9a–h and S10) | |
|---|---|---|---|---|---|---|---|---|
| Rhythm Control | Rate Control | |||||||
| All trials | 5 [20,21,22,25,26] | 815 | 6.6 ± 0.7 | 6.3 ± 0.7 | −0.8 days (−3.0 to +1.4) | 0.47 | 97% | Very low Skewed distribution, risk of bias, imprecision |
| Amiodarone-based rhythm control | 3 [20,24,25] | 723 | 6.1 ± 0.6 | 5.7 ± 0.6 | 0.5 days (−1.5 to +2.5) | 0.63 | 95% | |
| Nonamiodarone-based rhythm control | 2 [21,22] | 92 | 9.8 ± 1.3 | 12.6 ± 1.3 | −3.1 days (−6.2 to +0.1) | 0.06 | 64% | |
| Group | N Studies (References) | Number of Patients with Events/Number of Patients at Risk | Relative Risk | Quality of Evidence Reason for Judgement (Supplementary Files S7, S9 and S10) | |||
|---|---|---|---|---|---|---|---|
| Rhythm Control | Rate Control | Risk Ratio (95% CI) | p-Value | I2 | |||
| AF recurrence | |||||||
| AF recurrence within one week | 8 [19,20,21,22,23,24,25,26] | 79/605 | 51/451 | 1.1 (0.6–1.9) | 0.76 | 54% | Low Imprecision, risk of bias |
| AF recurrence up to one month | 3 [21,25,26] | 18/312 | 19/311 | 0.9 (0.5–1.8) | 0.84 | 0% | |
| AF recurrence up to three months | 3 [21,24,25] | 5/348 | 5/315 | 1.0 (0.3–3.4) | 0.95 | 0% | |
| Mortality | |||||||
| All studies | 5 [20,21,22,25,26] | 16/419 | 9/396 | 1.6 (0.7–3.5) | 0.24 | 0% | |
| Amiodarone-based rhythm control | 3 [20,25,26] | 14/360 | 9/363 | 1.5 (0.7–3.4) | 0.33 | 0% | Low Imprecision, risk of bias |
| NonAmiodarone-based rhythm control | 2 [21,22] | 2/57 | 0/35 | 4.3 (0.2–85.0) | 0.34 | N/A | |
| No significant subgroup differences for mortality (p = 0.51) | |||||||
| Stroke | |||||||
| All studies | 3 [22,25,26] | 4/297 | 6/318 | 0.7 (0.1–4.6) | 0.73 | 44% | Very low Very serious imprecision, risk of bias |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Belley-Coté, E.P.; Qiu, Y.; Belesiotis, P.; Tao, B.; Wolf, A.; Kaur, H.; Ibrahim, A.; Wong, J.A.; Wang, M.K.; et al. Rhythm vs. Rate Control in Patients with Postoperative Atrial Fibrillation after Cardiac Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4534. https://doi.org/10.3390/jcm12134534
Ahmed M, Belley-Coté EP, Qiu Y, Belesiotis P, Tao B, Wolf A, Kaur H, Ibrahim A, Wong JA, Wang MK, et al. Rhythm vs. Rate Control in Patients with Postoperative Atrial Fibrillation after Cardiac Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(13):4534. https://doi.org/10.3390/jcm12134534
Chicago/Turabian StyleAhmed, Muneeb, Emilie P. Belley-Coté, Yuan Qiu, Peter Belesiotis, Brendan Tao, Alex Wolf, Hargun Kaur, Alex Ibrahim, Jorge A. Wong, Michael K. Wang, and et al. 2023. "Rhythm vs. Rate Control in Patients with Postoperative Atrial Fibrillation after Cardiac Surgery: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 13: 4534. https://doi.org/10.3390/jcm12134534