Elective Endovascular Aneurysm Repair (EVAR) for the Treatment of Infrarenal Abdominal Aortic Aneurysms of 5.0–5.5 cm: Differences between Men and Women
Abstract
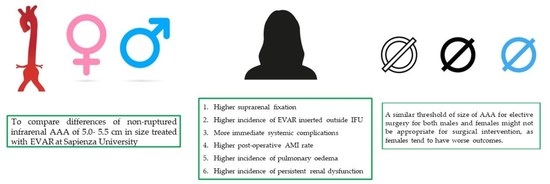
:1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Variables Considered
2.4. Preoperative Work-Up and Surgical Procedure
2.5. Postoperative Course and Surgical Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Postoperative Course
3.3. Mortality
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm |
| ASA | American Society of Anesthesiologists |
| COPD | chronic obstructive pulmonary disease |
| CDUS | color duplex ultrasound |
| CT | computed tomography |
| IMA | acute myocardial infarction |
| EVAR | endovascular aneurysm repair |
| MIP | Maximum-intensity projection images |
| OS | open surgery |
References
- Deery, S.E.; Soden, P.A.; Zettervall, S.L.; Shean, K.E.; Bodewes, T.C.F.; Pothof, A.B.; Lo, R.C.; Schermerhorn, M.L. Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J. Vasc. Surg. 2017, 65, 1006–1013. [Google Scholar] [CrossRef] [Green Version]
- Lo, R.C.; Bensley, R.P.; Hamdan, A.D.; Wyers, M.; Adams, J.E.; Schermerhorn, M.L. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J. Vasc. Surg. 2013, 57, 1261–1268.e5. [Google Scholar] [CrossRef] [Green Version]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [Green Version]
- Malayala, S.V.; Raza, A.; Vanaparthy, R. Gender-Based Differences in Abdominal Aortic Aneurysm Rupture: A Retrospective Study. J. Clin. Med. Res. 2020, 12, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Kontopodis, N.; Pantidis, D.; Dedes, A.; Daskalakis, N.; Ioannou, C.V. The–Not So–Solid 5.5 cm Threshold for Abdominal Aortic Aneurysm Repair: Facts, Misinterpretations, and Future Directions. Front. Surg. 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, P.; De Rango, P.; Verzini, F.; Parlani, G.; Romano, L.; Cieri, E.; CAESAR Trial Group. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): Results from a randomised trial. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Ouriel, K.; Clair, D.G.; Kent, K.C.; Zarins, C.K. Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J. Vasc. Surg. 2010, 51, 108–1087. [Google Scholar] [CrossRef] [Green Version]
- Bellini, M.I.; Adair, A.; Fotopoulou, C.; Graham, Y.; Hutson, A.; McNally, S.; Mohan, H.; Vig, S.; Parks, R.; Papalois, V. Changing the norm towards gender equity in surgery: The women in surgery working group of the Association of Surgeons of Great Britain and Ireland’s perspective. J. R. Soc. Med. 2019, 112, 325–329. [Google Scholar] [CrossRef]
- Steinberg, J.R.; Turner, B.E.; Weeks, B.T.; Magnani, C.J.; Wong, B.O.; Rodriguez, F.; Yee, L.M.; Cullen, M.R. Analysis of Female Enrollment and Participant Sex by Burden of Disease in US Clinical Trials Between 2000 and 2020. JAMA Netw. Open 2021, 4, e2113749. [Google Scholar] [CrossRef]
- Prinssen, M.; Verhoeven, E.L.; Buth, J.; Cuypers, P.W.; van Sambeek, M.R.; Balm, R.; Buskens, E.; Grobbee, D.E.; Blankensteijn, J.D. A Randomized Trial Comparing Conventional and Endovascular Repair of Abdominal Aortic Aneurysms. N. Engl. J. Med. 2004, 351, 1607–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuntz, M.; Audibert, C.; Su, Z. Persisting disparities between sexes in outcomes of ruptured abdominal aortic aneurysm hospitalizations. Sci. Rep. 2017, 7, 17994. [Google Scholar] [CrossRef] [Green Version]
- Cote, C.L.; Jessula, S.; Kim, Y.; Cooper, M.; McDougall, G.; Casey, P.; Dua, A.; Lee, M.S.; Smith, M.; Herman, C. Trends in Incidence of Abdominal Aortic Aneurysm Rupture, Repair and Mortality in Nova Scotia. Ann. Vasc. Surg. 2022. [Google Scholar] [CrossRef]
- Smedile, G.; Bellini, M.I.; Iaria, G.; Castrucci, T.; De Luca, L.; Leporelli, P.; Booth, C.; Orlando, G.; Tisone, G. Emergency Endovascular Repair in a Patient with Abdominal Aortic Aneurysm with Pelvic Transplant Kidneys: Case Report. Exp. Clin. Transplant. 2012, 10, 601–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scali, S.T.; Suckow, B.D.; Goodney, P.P.; de Guerre, L.E.; Schermerhorn, M.L.; Huber, T.S.; Upchurch, G.R.; Neal, D.; Columbo, J.A.; Kang, J.; et al. A significant proportion of current endovascular aortic aneurysm repair practice fails to meet Society for Vascular Surgery clinical practice guideline recommended abdominal aortic aneurysm diameter treatment thresholds in the Vascular Quality Initiative. J. Vasc. Surg. 2022, 75, 1234–1241.e1. [Google Scholar] [CrossRef]
- Witzemann, T.M.; Pardue, M.L. Exploring the Biological Contributions to Human Health: Does Sex Matter? National Academy Press: Cambridge, MA, USA, 2001; Volume 10, pp. 433–439. [Google Scholar]
- Forbes, T.L.; Lawlor, D.K.; DeRose, G.; Harris, K.A. Gender Differences in Relative Dilatation of Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2006, 20, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Solberg, S.; Singh, K.; Wilsgaard, T.; Jacobsen, B. Increased Growth Rate of Abdominal Aortic Aneurysms in Women. The Tromsø Study. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 145–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nana, P.; Dakis, K.; Brodis, A.; Spanos, K.; Kouvelos, G.; Eckstein, H.-H.; Giannoukas, A. A systematic review and meta-analysis on early mortality after abdominal aortic aneurysm repair in females in urgent and elective settings. J. Vasc. Surg. 2022, 75, 1082–1088.e6. [Google Scholar] [CrossRef]
- Ulug, P.; Sweeting, M.J.; von Allmen, R.S.; Thompson, S.G.; Powell, J.T.; Jones, E.; Bown, M.J.; Glover, M.J.; Michaels, J. Morphological suitability for endovascular repair, non-intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: Systematic reviews with meta-analysis. Lancet 2017, 389, 2482–2491. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, Y.; Zhao, J.; Chen, X.; Wang, J.; Ma, Y.; Huang, B.; Yuan, D.; Du, X. Systematic review and meta-analysis of sex differences in outcomes after endovascular aneurysm repair for infrarenal abdominal aortic aneurysm. J. Vasc. Surg. 2020, 71, 283–296.e4. [Google Scholar] [CrossRef] [Green Version]
- Jashari, F.; Ibrahimi, P.; Nicoll, R.; Bajraktari, G.; Wester, P.; Henein, M.Y. Coronary and carotid atherosclerosis: Similarities and differences. Atherosclerosis 2013, 227, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Bytyçi, I.; Shenouda, R.; Wester, P.; Henein, M.Y. Carotid Atherosclerosis in Predicting Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2021, 41, e224–e237. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Sweeting, M.J.; Powell, J.T.; Greenhalgh, R.M. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): A randomised controlled trial. Lancet 2016, 388, 2366–2374. [Google Scholar] [CrossRef] [Green Version]
- Greenhalgh, R. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: Randomised controlled trial. Lancet 2004, 364, 843–848. [Google Scholar] [CrossRef]
- Bryce, Y.; Kim, W.; Katzen, B.; Benenati, J.; Samuels, S. Outcomes over Time in Patients with Hostile Neck Anatomy Undergoing Endovascular Repair of Abdominal Aortic Aneurysm. J. Vasc. Interv. Radiol. 2018, 29, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Subedi, S.K.; Lee, A.M.; Landis, G.S. Suprarenal Fixation Barbs Can Induce Renal Artery Occlusion in Endovascular Aortic Aneurysm Repair. Ann. Vasc. Surg. 2010, 24, 113.e7–113.e10. [Google Scholar] [CrossRef] [PubMed]
- Mwipatayi, B.P.; Faraj, J.; Oshin, O.; Fitridge, R.; Wong, J.; Schermerhorn, M.L.; Becquemin, J.-P.; Boeckler, D.; Riambau, V.; Teijink, J.A.; et al. Endurant stent graft demonstrates promising outcomes in challenging abdominal aortic aneurysm anatomy. J. Vasc. Surg. 2021, 73, 69–80. [Google Scholar] [CrossRef]
- Barry, I.P.; Barns, M.; Verhoeven, E.; Wong, J.; Dubenec, S.; Heyligers, J.M.; Milner, R.; Shutze, W.P.; Bachoo, P.; Vlaskovky, P.; et al. Excluder Stent Graft-Related Outcomes in Patients with Aortic Neck Anatomy Outside of Instructions for Use (IFU) within the Global Registry for Endovascular Aortic Treatment (GREAT): Mid-term Follow-Up Results. Ann. Vasc. Surg. 2021, 76, 222–231. [Google Scholar] [CrossRef]
- Oliveira-Pinto, J.; Oliveira, N.; Bastos-Gonçalves, F.; Hoeks, S.; Van Rijn, M.J.; Raa, S.T.; Mansilha, A.; Verhagen, H.J. Long-term results of outside “instructions for use” EVAR. J. Cardiovasc. Surg. 2017, 58, 252–260. [Google Scholar] [CrossRef]
- Oliveira, N.F.; Gonçalves, F.B.; Ultee, K.; Pinto, J.P.; van Rijn, M.J.; Raa, S.T.; Mwipatayi, B.P.; Böckler, D.; Hoeks, S.E.; Verhagen, H.J. Patients with large neck diameter have a higher risk of type IA endoleaks and aneurysm rupture after standard endovascular aneurysm repair. J. Vasc. Surg. 2019, 69, 783–791. [Google Scholar] [CrossRef]
- Valdivia, A.R.; Beropoulis, E.; Pitoulias, G.; Pratesi, G.; Marcos, F.; Barbante, M.; Gandarias, C.; Torsello, G.; Bisdas, T.; Donas, K. Multicenter Registry about the Use of EndoAnchors in the Endovascular Repair of Abdominal Aortic Aneurysms with Hostile Neck Showed Successful but Delayed Endograft Sealing within Intraoperative Type Ia Endoleak Cases. Ann. Vasc. Surg. 2019, 60, 61–69. [Google Scholar] [CrossRef]
- Cuozzo, S.; Martinelli, O.; Brizzi, V.; Miceli, F.; Flora, F.; Sbarigia, E.; Gattuso, R. Early Experience with Ovation Alto Stent-Graft. Ann. Vasc. Surg. 2023, 88, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Keith, C.J.; Passman, M.A.; Gaffud, M.J.; Novak, Z.; Pearce, B.J.; Matthews, T.C.; Patterson, M.A.; Jordan, W.D. Comparison of outcomes following endovascular repair of abdominal aortic aneurysms based on size threshold. J. Vasc. Surg. 2013, 58, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group 1 (Females) | Group 2 (Males) | p |
|---|---|---|---|
| Preoperative AMI | 11 (25) | 17 (34) | ns |
| Arrhythmia | 12 (50) | 22 (24) | <0.001 |
| Arterial hypertension | 36 (81) | 38 (76) | ns |
| COPD | 25 (57) | 32 (64) | ns |
| Chronic kidney disease | 25 (57) | 11 (22) | 0.028 |
| Carotid disease | 31 (71) | 26 (52) | ns |
| Previous stroke | 6 (14) | 6 (12) | 0.01 |
| Previous carotid revascularization | 8 (18) | 4 (8) | <0.001 |
| Severe atherosclerotic disease of the aorta | 1 (2) | 38 (76) | 0.02 |
| Thoracic aortic aneurysm | 6 (14) | 6 (12) | ns |
| Chronic peripheral arterial disease | 4 (9) | 3 (6) | ns |
| Previous deep venous thrombosis | 10 (23) | 6 (12) | ns |
| Variable (mm) | Group 1 (Females) | Group 2 (Males) | p |
|---|---|---|---|
| AAA Axial diameter: mean ± st. dev. (range) | 52.68 ± 2.055 (49–56) | 53.18 ± 2.195 (49–57) | 0.722 |
| Diameter right iliac axis | 7.85 ± 0.838 (6–10) | 10.04 ± 2.98 (8–13) | <0.01 |
| Diameter left iliac axis | 8.1 ± 0.907 (6–10) | 10.96 ± 2.02 (8–16) | <0.01 |
| Diameter right femoral axis | 7.26 ± 0.741 (6–8) | 9.66 ± 1.2 (7–12) | <0.01 |
| Diameter left femoral axis | 7.1 ± 0.74 (6–8) | 9.64 ± 1.02 (7–12) | <0.01 |
| Neck length | 15.8 ± 4.3 (7–24) | 17.7 ± 5.3 (7–28) | <0.01 |
| Neck diameter | 25.8 ± 1.9 (21–30) | 22 ± 3 (16–28) | <0.01 |
| Neck angle | 49 ± 9.8 (27–68) | 40.1 ± 10 (23–64) | <0.01 |
| Variable | Group 1 (Females) | Group 2 (Males) | p |
|---|---|---|---|
| Length (mm) | p = 0.56 | ||
| ≥15 | 21 (47.7) | 35 (70.0) | |
| ≥10 length <15 | 21 (47.7) | 13 (26.0) | |
| ≤10 | 2 (4.5) | 2 (4) | |
| Total | 44 (100) | 50 (100) | |
| Diameter (mm) | p < 0.001 | ||
| <24 | 3 (6.8) | 35 (70.0) | |
| 24 ≤ diameter ≤ 26 | 20 (45.5) | 12 (24.0) | |
| >26 | 21 (47.7) | 3 (6.0) | |
| Total | 44 (100) | 50 (100) | |
| Angle (°) | p = 0.003 | ||
| <40° | 10 (22.7) | 28 (56.0) | |
| 40 ≤ angle ≤ 60 | 25 (56.8) | 18 (36.0) | |
| >60 | 9 (20.5) | 4 (8) | |
| Total | 44 (100) | 50 (100) |
| Variable | Total | Group 1 (Females) | Group 2 (Males) | p |
|---|---|---|---|---|
| EVAR suprarenal fixation | 43 (46) | 24 (55) | 19 (38) | 0.026 |
| EVAR inserted outside instruction for use * | 26 (28) | 16 (36) | 10 (20) | 0.035 |
| Endoleak | 35 (37) | 17 (39) | 18 (36) | ns |
| Vascular reintervention | 25 (27) | 11 (25) | 14 (28) | ns |
| ICU admission | 81 (86) | 37 (84) | 44 (88) | ns |
| Immediate systemic complications | 35 (37) | 21 (48) | 14 (28) | 0.021 |
| Post-operative AMI | 18 (19) | 14 (32) | 4 (8) | 0.001 |
| Arrhythmia | 17 (18) | 12 (27) | 5 (10) | 0.006 |
| Pulmonary oedema | 14 (15) | 12 (27) | 2 (4) | <0.001 |
| Pneumonia | 22 (23) | 11 (25) | 11 (22) | ns |
| Persistent renal dysfunction | 5 (5) | 4 (9) | 1 (2) | 0.029 |
| Cerebral ischemia | 4 (4) | 3 (7) | 1 (2) | ns |
| Arterial embolism of lower limbs | 5 (5) | 2 (5) | 3 (6) | ns |
| Deep venous thrombosis of lower limbs | 4 (4) | 3 (7) | 1 (2) | ns |
| EVAR-related (in-hospital) mortality | 7 (7) | 4 (9) | 3 (6) | ns |
| Perioperative (30 days) mortality | 8 (9) | 6 (14) | 2 (4) | ns |
| Long-term (5 years) mortality | 5 (5) | 2 (5) | 3 (6) | ns |
| Factor | Perioperative Mortality (p) | EVAR-Related Mortality (p) |
|---|---|---|
| Immediate systemic complications | 0.318 | 0.607 |
| AMI | 0.014 | 0.015 |
| Arrythmia | 0.248 | 0.049 |
| Pulmonary oedema | 0.034 | 0.015 |
| Pneumonia | 0.093 | 0.263 |
| Persistent renal dysfunction | 0.119 | <0.001 |
| Cerebral ischemia | 0.081 | <0.001 |
| Arterial embolism of lower limb | 0.046 | <0.001 |
| Deep venous thrombosis of lower limbs | 0.119 | <0.001 |
| Type of Graft | Total | Group 1 (Females) | Group 2 (Males) | p |
|---|---|---|---|---|
| Zenith endovascular grafts (Cook Medical, Bloomington, IN, USA) | 11 | 6 | 5 | ns |
| Gore Excluder AAA Endoprosthesis (W.L. Gore & Associates, Inc., Flagstaff, AZ, USA) | 19 | 9 | 10 | |
| Nellix (Endologix, Irvine, CA, USA) | 22 | 7 | 15 | |
| AFX endografts (Endologix Inc., Irvine, CA, USA) | 22 | 14 | 8 | |
| Ovation stent graft (Endologix, Irvine, CA, USA) | 4 | 2 | 2 | |
| Treovance stent grafts (Bolton Medical, Barcelona, Spain) | 2 | 2 | 0 | |
| Incraft aortic stent grafts (Cordis, Bridgewater, NJ, USA) | 5 | 3 | 2 | |
| Endurant stent graft (Medtronic, Minneapolis, MN, USA) | 9 | 1 | 8 | |
| Total | 94 | 44 | 50 |
| Women | Men | p | |
|---|---|---|---|
| No | 32 (73) | 42 (7) | ns |
| IA | 4 (9) | 5 (9) | |
| IB | 1 (2) | 2 (4) | |
| II | 7 (16) of which 5 (14) spontaneously resolved | 5 (9) of which 3 (5) spontaneously resolved | |
| III | 0 | 1 (2) |
| (a) | ||||
|---|---|---|---|---|
| In-Hospital Mortality | Mean | |||
| Estimate | Std. Error | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | |||
| Females | 53.291 | 3.187 | 47.045 | 59.537 |
| Males | 56.800 | 1.753 | 53.365 | 60.235 |
| Overall | 55.913 | 1.876 | 52.237 | 59.590 |
| (b) | ||||
| 30-Days Mortality | Mean | |||
| Estimate | Std. Error | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | |||
| Females | 55.851 | 2.862 | 50.242 | 61.460 |
| Males | 56.091 | 2.092 | 51.992 | 60.190 |
| Overall | 57.249 | 1.695 | 53.927 | 60.572 |
| (c) | ||||
| Long-Term Mortality | Mean | |||
| Estimate | Std. Error | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | |||
| Females | 59.636 | 1.478 | 56.740 | 62.532 |
| Males | 56.629 | 1.843 | 53.017 | 60.241 |
| Overall | 58.573 | 1.416 | 55.798 | 61.347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, O.; Cuozzo, S.; Miceli, F.; Gattuso, R.; D’Andrea, V.; Sapienza, P.; Bellini, M.I. Elective Endovascular Aneurysm Repair (EVAR) for the Treatment of Infrarenal Abdominal Aortic Aneurysms of 5.0–5.5 cm: Differences between Men and Women. J. Clin. Med. 2023, 12, 4364. https://doi.org/10.3390/jcm12134364
Martinelli O, Cuozzo S, Miceli F, Gattuso R, D’Andrea V, Sapienza P, Bellini MI. Elective Endovascular Aneurysm Repair (EVAR) for the Treatment of Infrarenal Abdominal Aortic Aneurysms of 5.0–5.5 cm: Differences between Men and Women. Journal of Clinical Medicine. 2023; 12(13):4364. https://doi.org/10.3390/jcm12134364
Chicago/Turabian StyleMartinelli, Ombretta, Simone Cuozzo, Francesca Miceli, Roberto Gattuso, Vito D’Andrea, Paolo Sapienza, and Maria Irene Bellini. 2023. "Elective Endovascular Aneurysm Repair (EVAR) for the Treatment of Infrarenal Abdominal Aortic Aneurysms of 5.0–5.5 cm: Differences between Men and Women" Journal of Clinical Medicine 12, no. 13: 4364. https://doi.org/10.3390/jcm12134364