Ocular Distribution of Brimonidine and Brinzolamide after Topical Instillation of a 0.1% Brimonidine Tartrate and 1% Brinzolamide Fixed-Combination Ophthalmic Suspension: An Interventional Study
Abstract
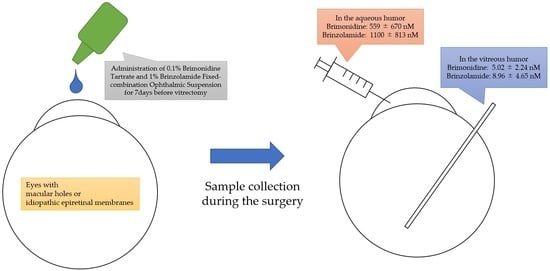
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Sample Collection
2.3. Sample Size
2.4. Drug Concentration Measurement
2.5. Primary Outcomes
2.6. Secondary Outcomes
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Primary Outcomes
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am. J. Ophthalmol. 1998, 126, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiji, A.; Leske, M.C.; Bengtsson, B. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar]
- Lusthaus, J.; Goldberg, I. Current management of glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef]
- MacIver, S.; Stout, N.; Ricci, O. New considerations for the clinical efficacy of old and new topical glaucoma medications. Clin. Exp. Optom. 2021, 104, 350–366. [Google Scholar] [CrossRef]
- Aihara, M. Prostanoid receptor agonists for glaucoma treatment. Jpn. J. Ophthalmol. 2021, 65, 581–590. [Google Scholar] [CrossRef]
- Gandolfi, S.; Cimino, L. Beta-adrenergic antagonists in the treatment of glaucoma. Eur. J. Ophthalmol. 2001, 11 (Suppl. S2), 63–66. [Google Scholar] [CrossRef]
- Stoner, A.; Harris, A.; Oddone, F.; Belamkar, A.; Vercellin, A.C.V.; Shin, J.; Januleviciene, I.; Siesky, B. Topical carbonic anhydrase inhibitors and glaucoma in 2021: Where do we stand? Br. J. Ophthalmol. 2022, 106, 1332–1337. [Google Scholar] [CrossRef]
- Oh, D.J.; Chen, J.L.; Vajaranant, T.S. Brimonidine tartrate for the treatment of glaucoma. Expert Opin. Pharmacother. 2019, 20, 115–122. [Google Scholar] [CrossRef]
- Tanna, A.P.; Johnson, M. Rho Kinase Inhibitors as a Novel Treatment for Glaucoma and Ocular Hypertension. Ophthalmology 2018, 125, 1741–1756. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.-Y.; Chen, C.-C.M.; Tseng, P.-C. Role of pilocarpine use following laser peripheral iridotomy in eyes with refractory acute angle closure glaucoma: A case report and literature review. Medicine 2022, 101, e29245. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Ichikawa, M.; Oku, H.; Shimazawa, M.; Araie, M. Bunazosin, a Selective α1 -Adrenoceptor Antagonist, as an Anti-glaucoma Drug: Effects on Ocular Circulation and Retinal Neuronal Damage. Cardiovasc. Drug Rev. 2005, 23, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Haria, M.; Spencer, C.M. Unoprostone (Isopropyl Unoprostone). Drugs Aging 1996, 9, 213–218, discussion 219–220. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, Y.; Inoue, T.; Shoji, N.; Nakamura, M.; Tanito, M.; Inoue, K.; Ishida, K.; Kurimoto, Y.; Suzuki, Y.; Chin, S.; et al. The Japan Glaucoma Society guidelines for glaucoma 5th edition. Jpn. J. Ophthalmol. 2023, 67, 189–254. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lindsley, K.; Rouse, B.; Hong, H.; Shi, Q.; Friedman, D.S.; Wormald, R.; Dickersin, K. Comparative effectiveness of firstline medications for primary open-angle glaucoma: A systematic review and network meta-analysis. Ophthalmology 2016, 123, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Garway-Heath, D.F.; Crabb, D.P.; Bunce, C. Latanoprost for open-angle glaucoma (UKGTS): A randomised, multicentre, placebo-controlled trial. Lancet 2015, 385, 1295–1304. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, B.; Heijl, A.; Johannesson, G.; Andersson-Geimer, S.; Aspberg, J.; Lindén, C. The Glaucoma Intensive Treatment Study (GITS), a randomized clinical trial: Design, methodology and baseline data. Acta Ophthalmol. 2018, 96, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, B.; Lindén, C.; Heijl, A.; Andersson-Geimer, S.; Aspberg, J.; Jóhannesson, G. The glaucoma intensive treatment study: Interim results from an ongoing longitudinal randomized clinical trial. Acta Ophthalmol. 2022, 100, e455–e462. [Google Scholar] [CrossRef]
- Gray, T.A.; Orton, L.C.; Henson, D.; Harper, R.; Waterman, H. Interventions for improving adherence to ocular hypotensive therapy. Cochrane Database Syst. Rev. 2009, 2, CD006132. [Google Scholar] [CrossRef] [Green Version]
- Fechtner, R.D.; Godfrey, D.G.; Budenz, D.; Stewart, J.A.; Stewart, W.C.; Jasek, M.C. Prevalence of Ocular Surface Complaints in Patients with Glaucoma Using Topical Intraocular Pressure-Lowering Medications. Cornea 2010, 29, 618–621. [Google Scholar] [CrossRef]
- Fukuchi, T.; Wakai, K.; Suda, K.; Nakatsue, T.; Sawada, H.; Hara, H.; Ueda, J.; Tanaka, T.; Yamada, A.; Abe, H. Incidence, severity and factors related to drug-induced keratoepitheliopathy with glaucoma medications. Clin. Ophthalmol. 2010, 4, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalicky, S.E.; Goldberg, I.; McCluskey, P. Ocular Surface Disease and Quality of Life in Patients with Glaucoma. Am. J. Ophthalmol. 2012, 153, 1–9.e2. [Google Scholar] [CrossRef] [PubMed]
- Djafari, F.; Lesk, M.R.; Harasymowycz, P.J.; Desjardins, D.; Lachaine, J. Determinants of Adherence to Glaucoma Medical Therapy in a Long-term Patient Population. Eur. J. Gastroenterol. Hepatol. 2009, 18, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Razeghinejad, M.R.; Sawchyn, A.K.; Katz, L.J. Fixed combinations of dorzolamide-timolol and brimonidine-timolol in the management of glaucoma. Expert Opin. Pharmacother. 2010, 11, 959–968. [Google Scholar] [CrossRef]
- Seibold, L.K.; DeWitt, P.E.; Kroehl, M.E.; Kahook, M.Y. The 24-Hour Effects of Brinzolamide/Brimonidine Fixed Combination and Timolol on Intraocular Pressure and Ocular Perfusion Pressure. J. Ocul. Pharmacol. Ther. 2017, 33, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Burke, J.A.; Potter, D.E. Ocular effects of a relatively selective alpha 2 agonist (UK-14, 304–318) in cats, rabbits and monkeys. Curr. Eye Res. 1986, 5, 665–676. [Google Scholar] [CrossRef]
- Lee, P.-Y.; Serle, J.B.; Podos, S.M.; Severin, C. Time course of the effect of UK 14304-18 (Brimonidine tartrate) on rabbit uveoscleral outflow. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1118. [Google Scholar]
- Lambert, W.S.; Ruiz, L.; Crish, S.D.; Wheeler, L.A.; Calkins, D.J. Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons. Mol. Neurodegener. 2011, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Yoles, E.; Wheeler, L.A.; Schwartz, M. Alpha2-adrenoreceptor agonists are neuroprotective in a rat model of optic nerve degeneration. Investig. Opthalmol. Vis. Sci. 1999, 40, 65–73. [Google Scholar]
- Wheeler, L.; WoldeMussie, E.; Lai, R. Role of Alpha-2 Agonists in Neuroprotection. Surv. Ophthalmol. 2003, 48 (Suppl. S1), S47–S51. [Google Scholar] [CrossRef]
- Krupin, T.; Liebmann, J.M.; Greenfield, D.S.; Ritch, R.; Gardiner, S. A Randomized Trial of Brimonidine Versus Timolol in Preserving Visual Function: Results From the Low-pressure Glaucoma Treatment Study. Am. J. Ophthalmol. 2011, 151, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Kawasaki, R.; Takahashi, H.; Maekawa, S.; Tsuda, S.; Omodaka, K.; Nakazawa, T. Effects of Brimonidine and Timolol on the Progression of Visual Field Defects in Open-angle Glaucoma: A Single-center Randomized Trial. J. Glaucoma 2019, 28, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Tomomatsu, T.; Matsumura, T.; Takihara, Y.; Kozai, S.; Arimura, S.; Yokota, S.; Inatani, M. Vitreous and aqueous concentrations of brimonidine following topical application of brimonidine tartrate 0.1% ophthalmic solution in humans. J. Ocul. Pharmacol. Ther. 2015, 31, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.R.; King, L.; Bartholomew, L.R. Vitreous Concentration of Topically Applied Brimonidine-Purite 0.15%. J. Ocul. Pharmacol. Ther. 2006, 22, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.R.; Nussdorf, J.D.; David, R.; Tyson, F.; Small, D.; Fellows, D. Vitreous concentration of topically applied brimonidine tartrate 0.2%. Ophthalmology 2001, 108, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.; Schwartz, M. Preclinical evaluation of brimonidine. Surv. Ophthalmol. 1996, 41 (Suppl. S1), S9–S18. [Google Scholar] [CrossRef]
- Orii, Y.; Kunikane, E.; Yamada, Y.; Morioka, M.; Iwasaki, K.; Arimura, S.; Mizuno, A.; Inatani, M. Brimonidine and timolol concentrations in the human vitreous and aqueous humors after topical instillation of a 0.1% brimonidine tartrate and 0.5% timolol fixed-combination ophthalmic solution: An interventional study. PLoS ONE 2022, 17, e0277313. [Google Scholar] [CrossRef]
- DeSantis, L. Preclinical Overview of Brinzolamide. Surv. Ophthalmol. 2000, 44 (Suppl. S2), S119–S129. [Google Scholar] [CrossRef]
- Katz, L.J. Twelve-Month Evaluation of Brimonidine-Purite Versus Brimonidine in Patients with Glaucoma or Ocular Hypertension. Eur. J. Gastroenterol. Hepatol. 2002, 11, 119–126. [Google Scholar] [CrossRef]
- Adkins, J.C.; Balfour, J.A. Brimonidine. A review of its pharmacological properties and clinical potential in the management of open-angle glaucoma and ocular hypertension. Drugs Aging 1998, 12, 225–241. [Google Scholar] [CrossRef]
- Maruyama, Y.; Ikeda, Y.; Yokoi, N.; Mori, K.; Kato, H.; Ueno, M.; Kinoshita, S.; Sotozono, C. Severe Corneal Disorders Developed After Brimonidine Tartrate Ophthalmic Solution Use. Cornea 2017, 36, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Cimolai, N. A review of neuropsychiatric adverse events from topical ophthalmic brimonidine. Hum. Exp. Toxicol. 2020, 39, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Saylor, M.; McLoon, L.K.; Harrison, A.R.; Lee, M.S. Experimental and Clinical Evidence for Brimonidine as an Optic Nerve and Retinal Neuroprotective Agent: An Evidence-Based Review. Arch. Ophthalmol. 2009, 127, 402–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, G.; Kunikane, E.; Shigemi, W.; Shinno, K.; Kozai, S.; Kurata, M.; Kawamura, A. Ocular and systemic pharmacokinetics of brimonidine and brinzolamide after topical administration in rabbits: Comparison between fixed-combination and single-drug formulations. Curr. Eye Res. 2021, 46, 380–386. [Google Scholar] [CrossRef]
- Mizuno, K.; Koide, T.; Shimada, S.; Mori, J.; Sawanobori, K.; Araie, M. Route of Penetration of Topically Instilled Nipradilol into the Ipsilateral Posterior Retina. Investig. Opthalmol. Vis. Sci. 2009, 50, 2839–2847. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Koide, T.; Saito, N.; Fujii, M.; Nagahara, M.; Tomidokoro, A.; Tamaki, Y.; Araie, M. Topical nipradilol: Effects on optic nerve head circulation in humans and periocular distribution in monkeys. Investig. Opthalmol. Vis. Sci. 2002, 43, 3243–3250. [Google Scholar]
- Grove, K.J.; Kansara, V.; Prentiss, M.; Long, D.; Mogi, M.; Kim, S.; Rudewicz, P.J. Application of Imaging Mass Spectrometry to Assess Ocular Drug Transit. SLAS Discov. Adv. Sci. Drug Discov. 2017, 22, 1239–1245. [Google Scholar] [CrossRef] [Green Version]
- Kalapesi, F.B.; Coroneo, M.T.; Hill, M.A. Human ganglion cells express the alpha-2 adrenergic receptor: Relevance to neuroprotection. Br. J. Ophthalmol. 2005, 89, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Woldemussie, E.; Wijono, M.; Pow, D. Localization of alpha 2 receptors in ocular tissues. Vis. Neurosci. 2007, 24, 745–756. [Google Scholar] [CrossRef]
- Aktas, Z.; Gürelik, G.; Akyürek, N. Neuroprotective effect of topically applied brimonidine tartrate 0.2% in endothelin-1-induced optic nerve ischaemia model. Clin. Exp. Ophthalmol. 2007, 35, 527–534. [Google Scholar] [CrossRef]
- Wheeler, L.; Lai, R.; WoldeMussie, E. From the Lab to the Clinic: Activation of an Alpha-2 agonist Pathway is Neuroprotective in Models of Retinal and Optic Nerve Injury. Eur. J. Ophthalmol. 1999, 9, S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.P.; Villegas-Pérez, M.P.; Sobrado-Calvo, P.; García-Avilés, A.; De Imperial, J.M.; Vidal-Sanz, M. Neuroprotective effects of alpha(2)-selective adrenergic agonists against ischemia-induced retinal ganglion cell death. Investig. Opthalmol. Vis. Sci. 2001, 42, 2074–2084. [Google Scholar]
- Danylkova, N.O.; Alcala, S.R.; Pomeranz, H.D. Neuroprotective effects of brimonidine treatment in a rodent model of ischemic optic neuropathy. Exp. Eye Res. 2007, 84, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Romano, G.L.; Eandi, C.M.; Toro, M.D.; Rejdak, R.; Di Benedetto, G.; Lazzara, F.; Bernardini, R.; Drago, F.; Cantarella, G.; et al. Brimonidine is Neuroprotective in Animal Paradigm of Retinal Ganglion Cell Damage. Front. Pharmacol. 2021, 12, 705405. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Harris-Cerruti, C.; Groner, Y.; Wheeler, L.A.; Schwartz, M.; Yoles, E. RGC death in mice after optic nerve crush injury: Oxidative stress and neuroprotection. Investig. Opthalmol. Vis. Sci. 2000, 41, 4169–4174. [Google Scholar]
- Wen, R.; Cheng, T.; Li, Y. Alpha 2-adrenergic agonists induce basic fibroblast growth factor expression in photoreceptors in vivo and ameliorate light damage. J. Neurosci. 1996, 16, 5986–5992. [Google Scholar] [CrossRef] [Green Version]
- Hernández, M.; Urcola, J.H.; Vecino, E. Retinal ganglion cell neuroprotection in a rat model of glaucoma following brimonidine, latanoprost or combined treatments. Exp. Eye Res. 2008, 86, 798–806. [Google Scholar] [CrossRef]
- WoldeMussie, E.; Ruiz, G.; Wijono, M.; Wheeler, L.A. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Investig. Opthalmol. Vis. Sci. 2001, 42, 2849–2855. [Google Scholar]
- Bakthavatchalam, M.; Lai, F.H.; Rong, S.S.; Ng, D.S.; Brelen, M.E. Treatment of cystoid macular edema secondary to retinitis pigmentosa: A systematic review. Surv. Ophthalmol. 2018, 63, 329–339. [Google Scholar] [CrossRef]
- Siesky, B.; Harris, A.; Brizendine, E.; Marques, C.; Loh, J.; Mackey, J.; Overton, J.; Netland, P. Literature Review and Meta-Analysis of Topical Carbonic Anhydrase Inhibitors and Ocular Blood Flow. Surv. Ophthalmol. 2009, 54, 33–46. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Characteristic | Value |
|---|---|
| Total (n) | 10 |
| Age, mean ± standard deviation (years) | 67.7 ± 10.0 |
| Sex, male/female (n) | 5/5 |
| Lens status, phakia/pseudophakia (n) | 9/1 |
| Diagnosis | |
| Idiopathic epiretinal membrane (n) | 9 |
| Macular hole (n) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orii, Y.; Kunikane, E.; Yamada, Y.; Morioka, M.; Iwasaki, K.; Arimura, S.; Mizuno, A.; Inatani, M. Ocular Distribution of Brimonidine and Brinzolamide after Topical Instillation of a 0.1% Brimonidine Tartrate and 1% Brinzolamide Fixed-Combination Ophthalmic Suspension: An Interventional Study. J. Clin. Med. 2023, 12, 4175. https://doi.org/10.3390/jcm12134175
Orii Y, Kunikane E, Yamada Y, Morioka M, Iwasaki K, Arimura S, Mizuno A, Inatani M. Ocular Distribution of Brimonidine and Brinzolamide after Topical Instillation of a 0.1% Brimonidine Tartrate and 1% Brinzolamide Fixed-Combination Ophthalmic Suspension: An Interventional Study. Journal of Clinical Medicine. 2023; 12(13):4175. https://doi.org/10.3390/jcm12134175
Chicago/Turabian StyleOrii, Yusuke, Eriko Kunikane, Yutaka Yamada, Masakazu Morioka, Kentaro Iwasaki, Shogo Arimura, Akemi Mizuno, and Masaru Inatani. 2023. "Ocular Distribution of Brimonidine and Brinzolamide after Topical Instillation of a 0.1% Brimonidine Tartrate and 1% Brinzolamide Fixed-Combination Ophthalmic Suspension: An Interventional Study" Journal of Clinical Medicine 12, no. 13: 4175. https://doi.org/10.3390/jcm12134175