A Multidimensional Approach to Assessing Factors Impacting Health-Related Quality of Life after Pediatric Traumatic Brain Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study and Study Participants
2.2. Ethical Approval
2.3. Procedure
2.4. Methods
2.4.1. Pre-Injury Characteristics
2.4.2. Injury-Related Characteristics
2.4.3. Post-Injury Outcomes
2.5. Statistical Considerations
2.6. Missing Data
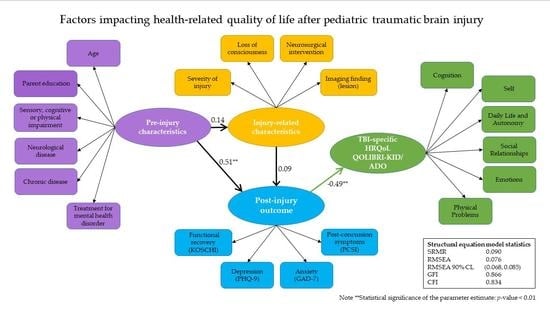
2.7. Path Analysis
- The ratio of chi-square statistics/degrees of freedom (χ2/df < 3) [57];
- The root mean square error of approximation (RMSEA; mediocre within 0.08–0.10, fair within 0.05–0.08, ideal fit < 0.05), as well as 90% confidence limits (adequate within 0.0001–0.090, ideal within 0.0001–0.054) [58];
- Standardized root mean square residual (SRMR; ideal < 0.055) [58];
- Goodness-of-fit index (GFI) and comparative fit index (CFI) (> 0.90 acceptable, > 0.94 very good fit) [58].
3. Results
3.1. Participants
3.2. Missing Data
3.3. Overview of the Analysis
3.3.1. The Initial and the Revised Measurement Model
3.3.2. The First and Revised Empirical SEM
4. Discussion
4.1. Limitations
4.2. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Instruments and Measures
Appendix A.1. Post-Injury Outcomes
Appendix A.1.1. Kings Outcome Scale for Childhood Head Injury (KOSCHI)—Investigator Rated
Appendix A.1.2. Post-Concussion Symptom Inventory (PCSI-SR8/SR13)—Self-Reported by Children and Adolescents
Appendix A.1.3. Patient Health Questionnaire 9 (PHQ-9)—Parent Rated
Appendix A.1.4. Generalized Anxiety Disorder-7 (GAD-7)—Parent Rated
Appendix A.2. TBI-Specific HRQoL Measure
Quality of Life after Traumatic Brain Injury in Kids/Adolescents (QOLIBRI-KID/ADO)—Self-Reported by Children and Adolescents
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic Brain Injury: Integrated Approaches to Improve Prevention, Clinical Care, and Research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [Green Version]
- Max, J.E.; Keatley, E.; Wilde, E.A.; Bigler, E.D.; Schachar, R.J.; Saunders, A.E.; Ewing-Cobbs, L.; Chapman, S.B.; Dennis, M.; Yang, T.T.; et al. Depression in Children and Adolescents in the First 6 Months after Traumatic Brain Injury. Int. J. Dev. Neurosci. 2012, 30, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Max, J.E.; Lopez, A.; Wilde, E.A.; Bigler, E.D.; Schachar, R.J.; Saunders, A.; Ewing-Cobbs, L.; Chapman, S.B.; Yang, T.T.; Levin, H.S. Anxiety Disorders in Children and Adolescents in the Second Six Months after Traumatic Brain Injury. J. Pediatr. Rehabil. Med. 2015, 8, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Olsson, K.A.; Lloyd, O.T.; LeBrocque, R.M.; McKinlay, L.; Anderson, V.A.; Kenardy, J.A. Predictors of Child Post-Concussion Symptoms at 6 and 18 Months Following Mild Traumatic Brain Injury. Brain Inj. 2013, 27, 145–157. [Google Scholar] [CrossRef]
- Connolly, E.J.; McCormick, B.F. Mild Traumatic Brain Injury and Psychopathology in Adolescence: Evidence from the Project on Human Development in Chicago Neighborhoods. J. Adolesc. Health 2019, 65, 79–85. [Google Scholar] [CrossRef]
- Han, P.P.; Holbrook, T.L.; Sise, M.J.; Sack, D.I.; Sise, C.B.; Hoyt, D.B.; Coimbra, R.; Potenza, B.; Anderson, J.P. Postinjury Depression Is a Serious Complication in Adolescents after Major Trauma: Injury Severity and Injury-Event Factors Predict Depression and Long-Term Quality of Life Deficits. J. Trauma Acute Care Surg. 2011, 70, 923–930. [Google Scholar] [CrossRef]
- Polinder, S.; Cnossen, M.C.; Real, R.G.L.; Covic, A.; Gorbunova, A.; Voormolen, D.C.; Master, C.L.; Haagsma, J.A.; Diaz-Arrastia, R.; von Steinbuechel, N. A Multidimensional Approach to Post-Concussion Symptoms in Mild Traumatic Brain Injury. Front. Neurol. 2018, 9, 1113. [Google Scholar] [CrossRef] [Green Version]
- Yeates, K.O. Predicting Postconcussive Symptoms after Mild Traumatic Brain Injury in Children and Adolescents. In Concussions in Athletics; Slobounov, S., Sebastianelli, W., Eds.; Springer: New York, NY, USA, 2014; pp. 273–287. [Google Scholar] [CrossRef]
- Chrisman, S.P.; Richardson, L.P. Prevalence of Diagnosed Depression in Adolescents with History of Concussion. J. Adolesc. Health 2014, 54, 582–586. [Google Scholar] [CrossRef] [Green Version]
- Andruszkow, H.; Deniz, E.; Urner, J.; Probst, C.; Grün, O.; Lohse, R.; Frink, M.; Krettek, C.; Zeckey, C.; Hildebrand, F. Physical and Psychological Long-Term Outcome after Traumatic Brain Injury in Children and Adult Patients. Health Qual. Life Outcomes 2014, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- McKinlay, A.; Ligteringen, V.; Than, M. A Comparison of Concussive Symptoms Reported by Parents for Preschool versus School-Aged Children. J. Head Trauma Rehabil. 2014, 29, 233–238. [Google Scholar] [CrossRef]
- Zemek, R.; Barrowman, N.; Freedman, S.B.; Gravel, J.; Gagnon, I.; McGahern, C.; Aglipay, M.; Sangha, G.; Boutis, K.; Beer, D.; et al. Clinical Risk Score for Persistent Postconcussion Symptoms Among Children with Acute Concussion in the ED. JAMA 2016, 315, 12. [Google Scholar] [CrossRef] [Green Version]
- McNally, K.A.; Bangert, B.; Dietrich, A.; Nuss, K.; Rusin, J.; Wright, M.; Taylor, H.G.; Yeates, K.O. Injury versus Noninjury Factors as Predictors of Postconcussive Symptoms Following Mild Traumatic Brain Injury in Children. Neuropsychology 2013, 27, 1. [Google Scholar] [CrossRef] [Green Version]
- Taylor, H.G.; Dietrich, A.; Nuss, K.; Wright, M.; Rusin, J.; Bangert, B.; Minich, N.; Yeates, K.O. Post-Concussive Symptoms in Children with Mild Traumatic Brain Injury. Neuropsychology 2010, 24, 148. [Google Scholar] [CrossRef] [Green Version]
- Dillard, C.; Ditchman, N.; Nersessova, K.; Foster, N.; Wehman, P.; West, M.; Riedlinger, B.; Monasterio, E.; Shaw, B.; Neblett, J. Post-Concussion Symptoms in Mild Traumatic Brain Injury: Findings from a Paediatric Outpatient Clinic. Disabil. Rehabil. 2017, 39, 544–550. [Google Scholar] [CrossRef]
- Max, J.E.; Pardo, D.; Hanten, G.; Schachar, R.J.; Saunders, A.E.; Ewing-Cobbs, L.; Chapman, S.B.; Dennis, M.; Wilde, E.A.; Bigler, E.D. Psychiatric Disorders in Children and Adolescents Six-to-Twelve Months after Mild Traumatic Brain Injury. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 272–282. [Google Scholar] [CrossRef]
- Calvert, S.; Miller, H.E.; Curran, A.; Hameed, B.; McCarter, R.; Edwards, R.J.; Hunt, L.; Sharples, P.M. The King’s Outcome Scale for Childhood Head Injury and Injury Severity and Outcome Measures in Children with Traumatic Brain Injury. Dev. Med. Child Neurol. 2008, 50, 426–431. [Google Scholar] [CrossRef]
- Anderson, V.; Catroppa, C.; Morse, S.; Haritou, F.; Rosenfeld, J. Recovery of Intellectual Ability Following Traumatic Brain Injury in Childhood: Impact of Injury Severity and Age at Injury. Pediatr. Neurosurg. 2000, 32, 282–290. [Google Scholar] [CrossRef]
- Muscara, F.; Catroppa, C.; Eren, S.; Anderson, V. The Impact of Injury Severity on Long-Term Social Outcome Following Paediatric Traumatic Brain Injury. Neuropsychol. Rehabil. 2009, 19, 541–561. [Google Scholar] [CrossRef]
- Albicini, M.; McKinlay, A. A Systematic Review of Anxiety Disorders Following Mild, Moderate and Severe TBI in Children and Adolescents. Fresh Look Anxiety Disord. 2015, 199–224. [Google Scholar] [CrossRef] [Green Version]
- Di Battista, A.; Godfrey, C.; Soo, C.; Catroppa, C.; Anderson, V. Depression and Health Related Quality of Life in Adolescent Survivors of a Traumatic Brain Injury: A Pilot Study. PLoS ONE 2014, 9, e101842. [Google Scholar] [CrossRef]
- Max, J.E.; Ch, M.B.B.; Keatley, E.; Wilde, E.A.; Bigler, E.D.; Levin, H.S.; Schachar, R.J.; Saunders, A.; Ewing-Cobbs, L.; Chapman, S.B.; et al. Anxiety Disorders in Children and Adolescents in the First Six Months After Traumatic Brain Injury. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grados, M.; Slomine, B.; Gerring, J.; Vasa, R.; Bryan, N.; Denckla, M. Depth of Lesion Model in Children and Adolescents with Moderate to Severe Traumatic Brain Injury: Use of SPGR MRI to Predict Severity and Outcome. J. Neurol. Neurosurg. Psychiatry 2001, 70, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babikian, T.; Freier, M.C.; Tong, K.A.; Nickerson, J.P.; Wall, C.J.; Holshouser, B.A.; Burley, T.; Riggs, M.L.; Ashwal, S. Susceptibility Weighted Imaging: Neuropsychologic Outcome and Pediatric Head Injury. Pediatr. Neurol. 2005, 33, 184–194. [Google Scholar] [CrossRef]
- Barlow, K.M.; Crawford, S.; Stevenson, A.; Sandhu, S.S.; Belanger, F.; Dewey, D. Epidemiology of Postconcussion Syndrome in Pediatric Mild Traumatic Brain Injury. Pediatrics 2010, 126, e374–e381. [Google Scholar] [CrossRef]
- O’Connor, S.S.; Zatzick, D.F.; Wang, J.; Temkin, N.; Koepsell, T.D.; Jaffe, K.M.; Durbin, D.; Vavilala, M.S.; Dorsch, A.; Rivara, F.P. Association between Posttraumatic Stress, Depression, and Functional Impairments in Adolescents 24 Months after Traumatic Brain Injury. J. Trauma Stress 2012, 25, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Hendry, K.; Ownsworth, T.; Waters, A.M.; Jackson, M.; Lloyd, O. Investigation of Children and Adolescents’ Mood and Self-Concept after Acquired Brain Injury. Child Neuropsychol. 2020, 26, 1005–1025. [Google Scholar] [CrossRef]
- Rosema, S.; Muscara, F.; Anderson, V.; Godfrey, C.; Eren, S.; Catroppa, C. Agreement on and Predictors of Long-Term Psychosocial Development 16 Years Post-Childhood Traumatic Brain Injury. J Neurotrauma 2014, 31, 899–905. [Google Scholar] [CrossRef]
- Catroppa, C.; Anderson, V.A.; Morse, S.A.; Haritou, F.; Rosenfeld, J.V. Outcome and Predictors of Functional Recovery 5 Years Following Pediatric Traumatic Brain Injury (TBI). J. Pediatr. Psychol. 2008, 33, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Upton, P.; Lawford, J.; Eiser, C. Parent–Child Agreement across Child Health-Related Quality of Life Instruments: A Review of the Literature. Qual. Life Res. 2008, 17, 895–913. [Google Scholar] [CrossRef]
- Tonks, J.; Slater, A.; Frampton, I.; Wall, S.E.; Yates, P.; Williams, W.H. The Development of Emotion and Empathy Skills after Childhood Brain Injury. Dev. Med. Child Neurol. 2009, 51, 8–16. [Google Scholar] [CrossRef]
- Rosema, S.; Muscara, F.; Anderson, V.; Godfrey, C.; Hearps, S.; Catroppa, C. The Trajectory of Long-Term Psychosocial Development 16 Years Following Childhood Traumatic Brain Injury. J. Neurotrauma 2015, 32, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Catroppa, C.; Godfrey, C.; Rosenfeld, J.V.; Hearps, S.S.J.C.; Anderson, V.A. Functional Recovery Ten Years after Pediatric Traumatic Brain Injury: Outcomes and Predictors. J. Neurotrauma 2012, 29, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Von Steinbuechel, N.; Richter, S.; Morawetz, C.; Riemsma, R. Assessment of Subjective Health and Health-Related Quality of Life in Persons with Acquired or Degenerative Brain Injury. Curr. Opin. Neurol. 2005, 18, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Von Steinbuechel, N.; Covic, A.; Polinder, S.; Kohlmann, T.; Cepulyte, U.; Poinstingl, H.; Backhaus, J.; Bakx, W.; Bullinger, M.; Christensen, A.-L. Assessment of Health-Related Quality of Life after TBI: Comparison of a Disease-Specific (QOLIBRI) with a Generic (SF-36) Instrument. Behav. Neurol. 2016, 2016, 7928014. [Google Scholar] [CrossRef] [Green Version]
- LeBlond, E.; Smith-Paine, J.; Narad, M.; Wade, S.L.; Gardis, M.; Naresh, M.; Makoroff, K.; Rhine, T. Understanding the Relationship between Family Functioning and Health-Related Quality of Life in Very Young Children with Moderate-to-Severe TBI. Clin. Neuropsychol. 2021, 35, 868–884. [Google Scholar] [CrossRef]
- Ryan, N.P.; Noone, K.; Godfrey, C.; Botchway, E.N.; Catroppa, C.; Anderson, V. Young Adults’ Perspectives on Health-Related Quality of Life after Paediatric Traumatic Brain Injury: A Prospective Cohort Study. Ann. Phys. Rehabil. Med. 2019, 62, 342–350. [Google Scholar] [CrossRef]
- Russell, K.; Selci, E.; Black, B.; Ellis, M.J. Health-Related Quality of Life Following Adolescent Sports-Related Concussion or Fracture: A Prospective Cohort Study. J. Neurosurg. Pediatr. 2019, 23, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Novak, Z.; Aglipay, M.; Barrowman, N.; Yeates, K.O.; Beauchamp, M.H.; Gravel, J.; Freedman, S.B.; Gagnon, I.; Gioia, G.; Boutis, K.; et al. Association of Persistent Postconcussion Symptoms with Pediatric Quality of Life. JAMA Pediatr. 2016, 170, e162900. [Google Scholar] [CrossRef] [Green Version]
- Russell, K.; Selci, E.; Chu, S.; Fineblit, S.; Ritchie, L.; Ellis, M.J. Longitudinal Assessment of Health-Related Quality of Life Following Adolescent Sports-Related Concussion. J. Neurotrauma 2017, 34, 2147–2153. [Google Scholar] [CrossRef]
- Yeates, K.O. Mild Traumatic Brain Injury and Postconcussive Symptoms in Children and Adolescents. J. Int. Neuropsychol. Soc. 2010, 16, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Plourde, V.; Yeates, K.O.; Brooks, B.L. Predictors of Long-Term Psychosocial Functioning and Health-Related Quality of Life in Children and Adolescents with Prior Concussions. J. Int. Neuropsychol. Soc. 2018, 24, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundfrom, D.J.; Shaw, D.G. Tian Lu Ke Minimum Sample Size Recommendations for Conducting Factor Analyses. Int. J. Test. 2005, 5, 159–168. [Google Scholar] [CrossRef]
- Havighurst, R.J. Developmental Tasks and Education; David McKay: New York, NY, USA, 1972. [Google Scholar]
- Varni, J.W. PedsQL TM (Pediatric Quality of Life Inventory TM). Available online: https://www.pedsql.org/score.html (accessed on 6 July 2022).
- Crouchman, M.; Rossiter, L.; Colaco, T.; Forsyth, R. A Practical Outcome Scale for Paediatric Head Injury. Arch. Dis. Child. 2001, 84, 120. [Google Scholar] [CrossRef] [Green Version]
- Sady, M.D.; Vaughan, C.G.; Gioia, G.A. Psychometric Characteristics of the Postconcussion Symptom Inventory in Children and Adolescents. Arch. Clin. Neuropsychol. 2014, 29, 348–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Zeldovich, M.; Cunitz, K.; Greving, S.; Muehlan, H.; Bockhop, F.; Krenz, U.; Timmermann, D.; Koerte, I.K.; Rojczyk, P.; Roediger, M.; et al. Psychometric Properties of the German Version of the Quality of Life After Brain Injury Scale for Kids and Adolescents (QOLIBRI-KID/ADO) Using Item Response Theory Framework: Results from the Pilot Study. J. Clin. Med. 2023, 12, 3716. [Google Scholar] [CrossRef]
- Gioia, G.; Vaughan, C.; Sady, M. Technical Manual for the Post Concussion Symptom InventoryTM–2 (PCSI-2); PAR: Lutz, FL, USA, 2019. [Google Scholar]
- O’Rourke, N.; O’Rourke, N.H.L.; Hatcher, L. A Step-by-Step Approach to Using SAS for Factor Analysis and Structural Equation Modeling, 2nd ed.; SAS Press: Cary, NC, USA, 2013; ISBN 978-1-59994-230-8. [Google Scholar]
- Nunnally, J. Psychometric Theory, 2nd ed.; McGraw: New York, NY, USA, 1978. [Google Scholar]
- Fornell, C.; Larcker, D.F. Evaluating Structural Equation Models with Unobservable Variables and Measurement Error. J. Mark. Res. 1981, 18, 39–50. [Google Scholar] [CrossRef]
- Hoyle, R.H.; Isherwood, J.C. Reporting Results from Structural Equation Modeling Analyses in Archives of Scientific Psychology. Arch. Sci. Psychol. 2013, 1, 14. [Google Scholar] [CrossRef]
- Chen, F.; Curran, P.J.; Bollen, K.A.; Kirby, J.; Paxton, P. An Empirical Evaluation of the Use of Fixed Cutoff Points in RMSEA Test Statistic in Structural Equation Models. Sociol. Methods Res. 2008, 36, 462–494. [Google Scholar] [CrossRef] [Green Version]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 4th ed.; Guilford Publications: New York, NY, USA, 2015; ISBN 978-1-4625-2335-1. [Google Scholar]
- Hu, L.; Bentler, P.M. Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria versus New Alternatives. Struct. Equ. Model. Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Schachar, R.J.; Park, L.S.; Dennis, M. Mental Health Implications of Traumatic Brain Injury (TBI) in Children and Youth. J. Can. Acad. Child Adolesc. Psychiat. J. Académie Canadienne Psychiatrie L’enfant L’adolescent 2015, 24, 100–108. [Google Scholar]
- Durish, C.L.; Pereverseff, R.S.; Yeates, K.O. Depression and Depressive Symptoms in Pediatric Traumatic Brain Injury: A Scoping Review. J. Head Trauma Rehabil. 2018, 33, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Ochi, M.; Fujiwara, T.; Mizuki, R.; Kawakami, N. World Mental Health Japan Survey Group Association of Socioeconomic Status in Childhood with Major Depression and Generalized Anxiety Disorder: Results from the World Mental Health Japan Survey 2002–2006. BMC Public Health 2014, 14, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinidad, S.; Kotagal, M. Socioeconomic Factors and Pediatric Injury. Curr. Trauma Rep. 2023, 9, 47–55. [Google Scholar] [CrossRef]
- Pelletier, J.H.; Rakkar, J.; Simon, D.; Au, A.K.; Fuhrman, D.Y.; Clark, R.S.B.; Kochanek, P.M.; Horvat, C.M. Association between Pediatric TBI Mortality and Median Family Income in the United States: A Retrospective Cohort Study. Lancet Reg. Health Am. 2022, 5, 100164. [Google Scholar] [CrossRef]
- Ewing-Cobbs, L.; Montroy, J.J.; Clark, A.E.; Holubkov, R.; Cox, C.S.; Keenan, H.T. As Time Goes by: Understanding Child and Family Factors Shaping Behavioral Outcomes After Traumatic Brain Injury. Front. Neurol. 2021, 12, 687740. [Google Scholar] [CrossRef]
- Arambula, S.E.; Reinl, E.L.; El Demerdash, N.; McCarthy, M.M.; Robertson, C.L. Sex Differences in Pediatric Traumatic Brain Injury. Exp. Neurol. 2019, 317, 168–179. [Google Scholar] [CrossRef]
- Tsai, F.-J.; Huang, Y.-H.; Liu, H.-C.; Huang, K.-Y.; Huang, Y.-H.; Liu, S.-I. Patient Health Questionnaire for School-Based Depression Screening Among Chinese Adolescents. Pediatrics 2014, 133, e402–e409. [Google Scholar] [CrossRef] [Green Version]
- Câmara-Costa, H.; Opatowski, M.; Francillette, L.; Toure, H.; Brugel, D.; Laurent-Vannier, A.; Meyer, P.; Watier, L.; Dellatolas, G.; Chevignard, M. Self- and Parent-Reported Quality of Life 7 Years after Severe Childhood Traumatic Brain Injury in the Traumatisme Grave de l’Enfant Cohort: Associations with Objective and Subjective Factors and Outcomes. Qual. Life Res. 2020, 29, 515–528. [Google Scholar] [CrossRef]
- Helmrich, I.R.R.; Czeiter, E.; Amrein, K.; Büki, A.; Lingsma, H.F.; Menon, D.K.; Mondello, S.; Steyerberg, E.W.; von Steinbüchel, N.; Wang, K.K. Incremental Prognostic Value of Acute Serum Biomarkers for Functional Outcome after Traumatic Brain Injury (CENTER-TBI): An Observational Cohort Study. Lancet Neurol. 2022, 21, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Lavinio, A.; Prisco, L. Delirium and Agitation in Traumatic Brain Injury Patients: An Update on Pathological Hypotheses and Treatment Options. Minerva Anestesiol. 2018, 84, S0375–S9393. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.; Ford, T.J.; Karl, A.; Reynolds, S.; Limond, J.; Adlam, A.-L.R. Mood Disorders in Young People with Acquired Brain Injury: An Integrated Model. Front. Hum. Neurosci. 2022, 16, 835897. [Google Scholar] [CrossRef] [PubMed]
- Von Steinbuechel, N.; Wilson, L.; Gibbons, H.; Hawthorne, G.; Höfer, S.; Schmidt, S.; Bullinger, M.; Maas, A.; Neugebauer, E.; Powell, J.; et al. Quality of Life after Brain Injury (QOLIBRI): Scale Validity and Correlates of Quality of Life. J. Neurotrauma 2010, 27, 1157–1165. [Google Scholar] [CrossRef] [PubMed]



| Children’s Characteristics | KID (n = 152) | ADO (n = 148) | Total (n = 300) | (Chi-Square, df) p-Value |
|---|---|---|---|---|
| Pre-Injury Characteristics | ||||
| Age (years) | N/A | |||
| Mean (SD) | 10.6 (1.40) | 15.2 (1.47) | 12.9 (2.72) | |
| Median (Range) | 10.5 (8.0–12.9) | 14.9 (13.0–17.9) | 12.9 (8.0–17.9) | |
| Sex N (%) | (0.44, 1) 0.508 | |||
| Female | 58 (38.2) | 62 (41.9) | 120 (40.0) | |
| Male | 93 (61.2) | 85 (57.4) | 178 (59.3) | |
| Missing | 1 (0.7) * | 1 (0.7) | 2 (0.7) | |
| Education (of both parents) N (%) | (0.91, 1) 0.340 | |||
| University | 97 (63.8) | 92 (62.2) | 189 (63.0) | |
| Other than university | 44 (28.9) | 53 (35.8) | 97 (32.3) | |
| Missing | 11 (7.9) | 3 (2.0) | 14 (4.7) | |
| Employment (of both parents) N (%) | (0.86, 1) 0.353 | |||
| Employed (>35 h/week) | 129 (84.9) | 133 (89.9) | 262 (87.3) | |
| Other than >35 h/week | 18 (11.8) | 13 (8.8) | 31 (10.3) | |
| Missing | 5 (3.3) | 2 (1.4) | 7 (2.3) | |
| Previous TBI N (%) | (0.01, 1) 0.941 | |||
| No | 126 (82.9) | 119 (80.4) | 245 (81.7) | |
| One or more TBIs | 26 (17.1) | 24 (16.2) | 50 (16.7) | |
| Missing | 0 (0.0) | 5 (3.4) | 5 (1.7) | |
| Prior cognitive, sensory, or physical impairment N (%) | (0.83, 1) 0.362 | |||
| No | 112 (76.7) | 102 (68.9) | 214 (71.3) | |
| Yes | 40 (26.3) | 46 (31.1) | 86 (28.7) | |
| Neurological disease N (%) | (0.39, 1) 0.535 | |||
| No | 143 (94.1) | 131 (88.5) | 274 (91.3) | |
| Yes | 9 (5.9) | 11 (7.4) | 20 (6.7) | |
| Missing | 0 (0.0) | 6 (4.1) | 6 (2.0) | |
| Chronic disease N (%) | (0.55, 1) 0.457 | |||
| No | 116 (76.3) | 103 (69.6) | 219 (73.0) | |
| Yes | 36 (23.7) | 39 (26.4) | 75 (25.0) | |
| Missing | 0 (0.0) | 6 (4.1) | 6 (2.0) | |
| Treatment for mental health disorder N (%) | (2.64, 1) 0.104 | |||
| No | 137 (90.1) | 130 (87.8) | 267 (89.0) | |
| Yes *** | 9 (5.9) | 17 (11.5) | 26 (8.7) | |
| Missing | 6 (3.9) | 1 (0.7) | 7 (2.3) | |
| Injury-Related Characteristics | ||||
| TBI severity N (%) | (0.57, 1) 0.452 | |||
| Mild | 106 (69.7) | 109 (73.6) | 215 (71.7) | |
| Moderate or severe | 46 (30.3) | 39 (26.4) | 85 (28.3) | |
| Imaging finding (lesion) N (%) | (0.05, 1) 0.819 | |||
| No | 105 (69.1) | 99 (66.9) | 204 (68.0) ** | |
| Yes | 43 (28.3) | 43 (29.1) | 86 (28.7%) | |
| Missing | 4 (2.6) | 6 (4.1) | 10 (3.3) | |
| Retrograde amnesia N (%) | (12.64, 1) <0.001 | |||
| No | 124 (81.6) | 93 (62.8) | 217 (72.3) | |
| Yes | 25 (16.4) | 50 (33.8) | 75 (25.0) | |
| Missing | 3 (2.0) | 5 (3.4) | 8 (2.7) | |
| Loss of consciousness N (%) | (4.09, 1) 0.043 | |||
| No | 111 (73.0) | 92 (62.2) | 203 (67.7) | |
| Yes | 40 (26.3) | 55 (37.2) | 95 (31.7) | |
| Missing | 1 (0.7) | 1 (0.7) | 2 (0.7) | |
| Neurosurgical intervention following injury N (%) | (0.32, 1) 0.571 | |||
| No | 127 (83.6) | 120 (81.1) | 247 (82.3) | |
| Yes | 24 (15.8) | 27 (18.2) | 51 (17.0) | |
| Missing | 1 (0.7) | 1 (0.7) | 2 (0.7) | |
| Time since injury (years) | ||||
| Mean (SD) | 4.2 (2.55) | 4.9 (2.95) | 4.5 (2.78) | N/A |
| Median (Range) | 3.7 (0.2, 9.4) | 4.5 (0.2, 10.2) | 4.1 (0.2, 10.2) | |
| Missing | 0 | 1 | 1 | |
| Post-Injury Outcome Measures | KID (n = 152) | ADO (n = 148) | Total (n = 300) | (Test Statistic, df) p-Value |
|---|---|---|---|---|
| KOSCHI N (%) | (8.55, 1) 0.004 | |||
| 3a, 3b, 4a, 4b | 8 (5.3) | 23 (15.5) | 31 (10.3) | |
| 5a, 5b | 144 (94.7) | 125 (84.5) | 269 (89.7) | |
| PCSI ª | ||||
| Mean (SD) | 5.0 (5.48) | 19.8 (18.02) | N/A | N/A |
| Median (Range) | 3.0 (0–27) | 13.5 (0–75) | ||
| Missing | 19 | 22 | ||
| GAD-7 | (0.85, 1) 0.358 | |||
| Mean (SD) | 3.6 (3.07) | 3.4 (3.56) | 3.5 (3.32) | |
| Median (Range) | 3.0 (0–13) | 2.0 (0–17) | 2.0 (0–17) | |
| Missing | 5 | 2 | 7 | |
| PHQ-9 | (1.73, 1) 0.188 | |||
| Mean (SD) | 3.9 (3.36) | 4.7 (4.19) | 4.3 (3.81) | |
| Median (Range) | 3.0 (0–17) | 4.0 (0–21) | 3.0 (0–21) | |
| Missing | 5 | 2 | 7 | |
| GAD-7 (as categorical) N (%) | N/A | |||
| None or minimal anxiety (0–4) | 98 (64.5) | 110 (74.3) | 208 (69.3) | |
| Mild anxiety (5–9) | 43 (28.3) | 24 (16.2) | 67 (22.3) | |
| Moderate to severe anxiety (≥10) | 6 (3.9) | 12 (8.1) | 18 (6.0) | |
| Missing | 5 (3.3) | 2 (1.4) | 7 (2.3) | |
| PHQ-9 (as categorical) N (%) | N/A | |||
| None or minimal depression (0–4) | 98 (64.5) | 89 (60.1) | 187 (62.3) | |
| Mild depression (5–9) | 38 (25.0) | 37 (25.0) | 75 (25.0) | |
| Moderate to severe depression (≥10) | 11 (7.0) | 20 (13.5) | 31 (10.3) | |
| Missing | 5 (3.3) | 2 (1.4) | 7 (2.30) |
| QOLIBRI-KID/ADO | KID (n = 152) | ADO (n = 148) | Total (n = 300) | (Chi-Square, df) p-Value |
|---|---|---|---|---|
| Total score | (10.39, 1) 0.001 | |||
| Mean (SD) | 76.9 (10.71) | 72.6 (11.46) | 74.8 (11.27) | |
| Median (Range) | 76.4 (43.6–96.4) | 73.6 (27.1–93.6) | 75.7 (27.1–96.4) | |
| Missing | 0 | 3 | 3 | |
| Cognition | (18.78, 1) <0.001 | |||
| Mean (SD) | 78.6 (11.64) | 71.7 (14.29) | 75.2 (13.45) | |
| Median (Range) | 78.6 (42.9–100) | 75.0 (25–100) | 75.0 (25–100) | |
| Self | (52.54, 1) <0.001 | |||
| Mean (SD) Missing | 86.3 (11.83) | 73.6 (16.41) | 80.1 (15.61) | |
| Median (Range) | 90.0 (40–100) | 75.0 (15–100) | 85.0 (15–100) | |
| Missing | 0 | 1 | 1 | |
| Daily life and autonomy | (11.37, 1) <0.001 | |||
| Mean (SD) Missing | 88.7 (10.20) 0 | 84.5 (12.24) 2 | 86.6 (11.42) 2 | |
| Median (Range) | 92.9 (42.9–100) | 89.3 (28.6–100) | 89.3 (28.6–100) | |
| Missing | 0 | 2 | 2 | |
| Social relationships | (13.04, 1) <0.001 | |||
| Mean (SD) | 84.0 (12.24) | 79.3 (12.85) | 81.7 (12.75) | |
| Median (Range) | 87.5 (37.5–100) | 79.2 (29.2–100) | 83.3 (29.2–100) | |
| Emotions | (0, 1) 0.997 | |||
| Mean (SD) | 52.6 (25.02) | 53.4 (23.08) | 53.0 (24.05) | |
| Median (Range) | 56.3 (0–100) | 50.0 (6.3–100) | 53.1 (0–100) | |
| Physical problems | (1.06, 1) 0.303 | |||
| Mean (SD) | 61.9 (23.47) | 65.2 (19.58) | 63.5 (21.66) | |
| Median (Range) | 62.5 (0–100) | 66.7 (8.3–100) | 66.7 (0–100) |
| University (n = 189) | Other than University (n = 97) | Missing (n = 14) | (χ2, df) p-Value | |
|---|---|---|---|---|
| QOLIBRI-KID/ADO Total | (3.07, 1) 0.080 | |||
| Mean (SD) | 75.6 (10.73) | 72.5 (12.36) | 78.4 (8.32) | |
| Median (Range) | 75.7 (47.9–96.4) | 73.6 (27.1–95) | 80.4 (62.1–90) | |
| Missing | 1 | 2 | 0 | |
| PHQ-9 | (7.95, 1) 0.005 | |||
| Mean (SD) | 3.9 (3.67) | 5.2 (4.01) | 2.8 (2.64) | |
| Median (Range) | 3.0 (0–21) | 4.0 (0–17) | 2.0 (0–9) | |
| Missing | 1 | 1 | 5 | |
| GAD-7 | (5.74, 1) 0.017 | |||
| Mean (SD) | 3.1 (3.03) | 4.3 (3.74) | 2.9 (3.06) | |
| Median (Range) | 2.0 (0–17) | 3.0 (0–16) | 2.0 (0–10) | |
| Missing | 1 | 1 | 5 | |
| PCSI Total (log z-transformed) | (3.15, 1) 0.076 | |||
| Mean (SD) | −0.1 (0.95) | 0.2 (1.08) | −0.2 (1.07) | |
| Median (Range) | 0.0 (−2.6–2.1) | 0.4 (−2.6–2.1) | 0.1 (−1.6–1.7) | |
| Missing | 18 | 21 | 2 |
| Measurement and SEMs for QOLIBRI-KID/ADO–FIML Method | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model Description | Fit Statistics | ||||||||
| χ2 | df (Ratio χ2/df) | p-Value | SRMR | RMSEA | RMSEA 90% CI | GFI | CFI | Difference between Models χ2 diff, df, p-Value | |
| 1. Initial measurement model (all variables in) | 571.2 | 246 (2.3) | <0.0001 | 0.0849 | 0.0664 | [0.0593, 0.0735] | 0.8598 | 0.8290 | |
| 2. Revised measurement model without non-significant paths | 452.2 | 164 (2.8) | <0.0001 | 0.0887 | 0.0765 | [0.0682, 0.0850] | 0.8664 | 0.8351 | |
| 3. First SEM | 456.0 | 165 (2.8) | <0.0001 | 0.0900 | 0.0767 | [0.0683, 0.0851] | 0.8659 | 0.8334 | 3.8, 1 df, 0.051 (Model 3–Model 2) |
| 4. Revised final SEM * | 456.2 | 166 (2.7) | <0.0001 | 0.0901 | 0.0763 | [0.0680, 0.0847] | 0.8660 | 0.8339 | 0.2, 1 df, 0.655 (Model 4–Model 3) 4.0, 2 df, 0.135 (Model 4–Model 2) |
| Construct and Indicators | Standardized Loading | t a | Reliability | Variance Extracted Estimate |
|---|---|---|---|---|
| Pre-injury characteristics | 0.49 b | 0.16 | ||
| Age | 0.24 | 3.31 | 0.06 | |
| Education | 0.30 | 4.04 | 0.09 | |
| Sensory, cognitive or physical impairment | 0.36 | 5.21 | 0.13 | |
| Neurological disease | 0.22 | 2.87 | 0.05 | |
| Chronic disease | 0.49 | 6.77 | 0.24 | |
| Treatment for mental health disorder | 0.61 | 8.87 | 0.37 | |
| Injury-related characteristics | 0.83 b | 0.57 | ||
| Imaging finding (lesion) | 0.86 | 36.97 | 0.75 | |
| Neurosurgical intervention | 0.70 | 20.65 | 0.49 | |
| LOC | 0.43 | 8.60 | 0.19 | |
| Severity of injury | 0.92 | 42.48 | 0.84 | |
| Post-injury outcome | 0.56 b | 0.38 | ||
| PCSI | 0.36 | 5.98 | 0.13 | |
| KOSCHI | −0.24 | −4.00 | 0.06 | |
| PHQ-9 | 0.88 | 26.26 | 0.78 | |
| GAD-7 | 0.75 | 21.39 | 0.57 | |
| TBI-specific HRQoL (QOLIBRI-KID/ADO) | 0.82 b | 0.45 | ||
| Cognition | 0.82 | 30.63 | 0.67 | |
| Self | 0.72 | 21.53 | 0.52 | |
| Daily life and autonomy | 0.79 | 27.56 | 0.62 | |
| Social relationships | 0.76 | 24.64 | 0.57 | |
| Emotions | 0.36 | 6.46 | 0.13 | |
| Physical problems | 0.41 | 7.74 | 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Steinbuechel, N.; Krenz, U.; Bockhop, F.; Koerte, I.K.; Timmermann, D.; Cunitz, K.; Zeldovich, M.; Andelic, N.; Rojczyk, P.; Bonfert, M.V.; et al. A Multidimensional Approach to Assessing Factors Impacting Health-Related Quality of Life after Pediatric Traumatic Brain Injury. J. Clin. Med. 2023, 12, 3895. https://doi.org/10.3390/jcm12123895
von Steinbuechel N, Krenz U, Bockhop F, Koerte IK, Timmermann D, Cunitz K, Zeldovich M, Andelic N, Rojczyk P, Bonfert MV, et al. A Multidimensional Approach to Assessing Factors Impacting Health-Related Quality of Life after Pediatric Traumatic Brain Injury. Journal of Clinical Medicine. 2023; 12(12):3895. https://doi.org/10.3390/jcm12123895
Chicago/Turabian Stylevon Steinbuechel, Nicole, Ugne Krenz, Fabian Bockhop, Inga K. Koerte, Dagmar Timmermann, Katrin Cunitz, Marina Zeldovich, Nada Andelic, Philine Rojczyk, Michaela Veronika Bonfert, and et al. 2023. "A Multidimensional Approach to Assessing Factors Impacting Health-Related Quality of Life after Pediatric Traumatic Brain Injury" Journal of Clinical Medicine 12, no. 12: 3895. https://doi.org/10.3390/jcm12123895