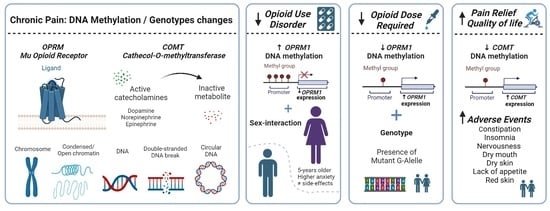
Sex Differences in Opioid Response Linked to OPRM1 and COMT genes DNA Methylation/Genotypes Changes in Patients with Chronic Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Data Collection
2.2.1. Clinical Outcomes
2.2.2. Pharmacology and Hospital Resources Use
2.3. Genetic/Epigenetic Data
2.3.1. Genotypes Analysis
2.3.2. DNA Methylation Analysis
2.4. Statistical Analysis
3. Results
3.1. Sex Differences in the Demographic and Clinical Data
3.2. DNA Methylation/Genotypes and Analgesic Response
3.2.1. Associations Linked to OPRM1 DNA Methylation
3.2.2. Associations Linked to COMT DNA Methylation
4. Discussion
4.1. OPRM1 DNA Methylation and Opioid Use Disorder
4.2. OPRM1 Methylation-Genotype Interaction in MEDD
4.3. COMT Methylation and Analgesic Response
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Primer | Sequence | CpG sites |
|---|---|---|
| OPRM1_F1 | 5′-GGATTGGTTTTTGTAAGAAATAGTAGG-3′ | |
| OPRM1_R1 | 5′-ATACRCCAAAACATCAATACAATTACTAAC-3′ | |
| OPRM1_S1 | 5′-AAGTTTYGGTGTTTTTGGTTA-3′ | CpG 7–11 |
| COMT_F1 | 5′-GTGGGGTTTTTGGGGTAGT-3′ | |
| COMT _R1 | 5′-ATCTAACCAACRCTCTCACCTCTCCC-3′ | |
| COMT _S1 | 5′-GGGTTTTTGGGGTAGTTA-3′ | CpG 37–42 |
| Total n = 250 | Females n = 125 | Males n = 125 | |
|---|---|---|---|
| Main opioid (%) | |||
| Buprenorphine | 5 | 2 | 9 |
| Fentanyl | 24 | 26 | 22 |
| Morphine | 8 | 5 | 11 |
| Oxycodone | 19 | 25 | 13 |
| Tapentadol | 31 | 29 | 33 |
| Tramadol | 12 | 14 | 11 |
References
- Louwies, T.; Greenwood-Van Meerveld, B. Sex Differences in the Epigenetic Regulation of Chronic Visceral Pain Following Unpredictable Early Life Stress. Neurogastroenterol. Motil. 2020, 32, e13751. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T. Epigenetic Effect of Environmental Factors on Neurodevelopmenal Disorders. Jpn. J. Hyg. 2016, 71, 200–207. [Google Scholar] [CrossRef]
- Sorge, R.E.; Totsch, S.K. Sex Differences in Pain. J. Neurosci. Res. 2017, 95, 1271–1281. [Google Scholar] [CrossRef]
- Osborne, V.; Serdarevic, M.; Crooke, H.; Striley, C.; Cottler, L.B. Non-Medical Opioid Use in Youth: Gender Differences in Risk Factors and Prevalence. Addict. Behav. 2017, 72, 114–119. [Google Scholar] [CrossRef]
- Nasser, S.A.; Afify, E.A. Sex Differences in Pain and Opioid Mediated Antinociception: Modulatory Role of Gonadal Hormones. Life Sci. 2019, 237, 116926. [Google Scholar] [CrossRef]
- Pieretti, S.; Di Giannuario, A.; Di Giovannandrea, R.; Marzoli, F.; Piccaro, G.; Minosi, P.; Aloisi, A.M. Gender Differences in Pain and Its Relief. Ann. Dell’istituto Super. Di Sanita 2016, 52, 184–189. [Google Scholar] [CrossRef]
- Pisanu, C.; Franconi, F.; Gessa, G.L.; Mameli, S.; Pisanu, G.M.; Campesi, I.; Leggio, L.; Agabio, R. Sex Differences in the Response to Opioids for Pain Relief: A Systematic Review and Meta-Analysis. Pharmacol. Res. 2019, 148, 104447. [Google Scholar] [CrossRef] [PubMed]
- Oertel, B.G.; Doehring, A.; Roskam, B.; Kettner, M.; Hackmann, N.; Ferreirós, N.; Schmidt, P.H.; Lötsch, J. Genetic-Epigenetic Interaction Modulates μ-Opioid Receptor Regulation. Hum. Mol. Genet. 2012, 21, 4751–4760. [Google Scholar] [CrossRef] [PubMed]
- Chlamydas, S.; Markouli, M.; Strepkos, D.; Piperi, C. Epigenetic Mechanisms Regulate Sex-Specific Bias in Disease Manifestations. J. Mol. Med. 2022, 100, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L. Sex, Gender, and Pain: A Review of Recent Clinical and Experimental Findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef]
- Mogil, J.S.; Bailey, A.L. Sex and Gender Differences in Pain and Analgesia. Prog. Brain Res. 2010, 186, 140–157. [Google Scholar] [CrossRef]
- Diatchenko, L.; Slade, G.D.; Nackley, A.G.; Bhalang, K.; Sigurdsson, A.; Belfer, I.; Goldman, D.; Xu, K.; Shabalina, S.A.; Shagin, D.; et al. Genetic Basis for Individual Variations in Pain Perception and the Development of a Chronic Pain Condition. Hum. Mol. Genet. 2005, 14, 135–143. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex Differences in Pain: A Brief Review of Clinical and Experimental Findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bagot, R.C.; Meaney, M.J. Epigenetics and the Biological Basis of Gene × Environment Interactions. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 752–771. [Google Scholar] [CrossRef]
- Owusu Obeng, A.; Hamadeh, I.; Smith, M. Review of Opioid Pharmacogenetics and Considerations for Pain Management. Pharmacotherapy 2017, 37, 1105–1121. [Google Scholar] [CrossRef]
- Yoshida, K.; Nishizawa, D.; Ide, S.; Ichinohe, T.; Fukuda, K.I.; Ikeda, K. A Pharmacogenetics Approach to Pain Management. Neuropsychopharmacol. Rep. 2018, 38, 2–8. [Google Scholar] [CrossRef]
- Muriel, J.; Margarit, C.; Barrachina, J.; Ballester, P.; Flor, A.; Morales, D.; Horga, J.F.; Fernández, E.; Peiró, A.M. Pharmacogenetics and Prediction of Adverse Events in Prescription Opioid Use Disorder Patients. Basic Clin. Pharmacol. Toxicol. 2019, 124, 439–448. [Google Scholar] [CrossRef]
- Sia, A.T.; Lim, Y.; Lim, E.C.P.; Ocampo, C.E.; Lim, W.Y.; Cheong, P.; Tan, E.C. Influence of Mu-Opioid Receptor Variant on Morphine Use and Self-Rated Pain Following Abdominal Hysterectomy. J. Pain 2013, 14, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, A.A.; Coller, J.K.; Barratt, D.T. Pharmacogenetics of Opioid Response. Clin. Pharmacol. Ther. 2015, 97, 125–127. [Google Scholar] [CrossRef]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Callaghan, J.T.; Samer, C.F.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin. Pharmacol. Ther. 2021, 110, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Swiergiel, A.H.; Juszczak, G.R.; Stankiewicz, A.M. Genetic and Epigenetic Mechanisms Linking Pain and Psychiatric Disorders. Pain Psychiatr. Disord. 2015, 30, 120–137. [Google Scholar]
- Polli, A.; Ickmans, K.; Godderis, L.; Nijs, J. When Environment Meets Genetics: A Clinical Review of the Epigenetics of Pain, Psychological Factors, and Physical Activity. Arch. Phys. Med. Rehabil. 2019, 100, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Planelles, B.; Margarit, C.; Ajo, R.; Sastre, Y.; Muriel, J.; Inda, M.-D.; Esteban, M.D.; Peiró, A.M. Health Benefits of an Adverse Events Reporting System for Chronic Pain Patients Using Long-Term Opioids. Acta Anaesthesiol. Scand. 2019, 63, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Margarit, C.; Roca, R.; Inda, M.D.; Muriel, J.; Ballester, P.; Moreu, R.; Conte, A.L.; Nunez, A.; Morales, D.; Peiro, A.M. Genetic Contribution in Low Back Pain: A Prospective Genetic Association Study. Pain Pract. 2019, 19, 836–847. [Google Scholar] [CrossRef]
- Barrachina, J.; Muriel, J.; Margarit, C.; Planelles, B.; Ballester, P.; Richart-Martínez, M.; Cutillas, E.; Zandonai, T.; Morales, D.; Peiró, A.M. Global Pain State Questionnaire: Reliability, Validity, and Gender Gap. Arch. Intern. Med. Res. 2021, 4, 91–113. [Google Scholar] [CrossRef]
- Snaith, R.P. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003, 1, 29. [Google Scholar] [CrossRef]
- Pergolizzi, J.; Böger, R.H.; Budd, K.; Dahan, A.; Erdine, S.; Hans, G.; Kress, H.G.; Langford, R.; Likar, R.; Raffa, R.B.; et al. Opioids and the Management of Chronic Severe Pain in the Elderly: Consensus Statement of an International Expert Panel with Focus on the Six Clinically Most Often Used World Health Organization Step III Opioids (Buprenorphine, Fentanyl, Hydromorphone, Methadone, Morphine, Oxycodone). Pain Pract. 2008, 8, 287–313. [Google Scholar] [CrossRef]
- Nielsen, D.A.; Yuferov, V.; Hamon, S.; Jackson, C.; Ho, A.; Ott, J.; Kreek, M.J. Increased OPRM1 DNA Methylation in Lymphocytes of Methadone-Maintained Former Heroin Addicts. Neuropsychopharmacology 2009, 34, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Chen, X.; Wu, N.; Shen, C.; Cui, H.; Du, W.; Zhang, Z.; Feng, M.; Liu, J.; Lin, S.; et al. Catechol-O-Methyltransferase Promoter Hypomethylation Is Associated with the Risk of Coronary Heart Disease. Exp. Ther. Med. 2016, 12, 3445–3449. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. Chapter 2—DNA Methylation and Cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [CrossRef]
- Viet, C.T.; Dang, D.; Aouizerat, B.E.; Miaskowski, C.; Ye, Y.; Viet, D.T.; Ono, K.; Schmidt, B.L. OPRM1 Methylation Contributes to Opioid Tolerance in Cancer Patients. J. Pain 2017, 18, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, G.; Asadikaram, G.; Akbari, H.; Nematollahi, M.H.; Abolhassani, M.; Shahabinejad, G.; Khodadadnejad, L.; Hashemi, M. Elevated Levels of DNA Methylation at the OPRM1 Promoter Region in Men with Opioid Use Disorder. Am. J. Drug Alcohol Abus. 2018, 44, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Korneliussen, T.; Albrechtsen, A.; Li, Y.; Wang, J. SNP Calling, Genotype Calling, and Sample Allele Frequency Estimation from New-Generation Sequencing Data. PLoS ONE 2012, 7, e37558. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Sierra, J.V.; Salgado García, F.I.; Brooks, J.H.; Derefinko, K.J.; Mozhui, K. Effect of Short-Term Prescription Opioids on DNA Methylation of the OPRM1 Promoter. Clin. Epigenetics 2020, 12, 76. [Google Scholar] [CrossRef]
- Kawarai, Y.; Jang, S.H.; Lee, S.; Millecamps, M.; Kang, H.M.; Gregoire, S.; Suzuki-Narita, M.; Ohtori, S.; Stone, L.S. Exercise Attenuates Low Back Pain and Alters Epigenetic Regulation in Intervertebral Discs in a Mouse Model. Spine J. 2021, 21, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, S.; Jang, S.H.; Szyf, M.; Stone, L.S. Prenatal Maternal Stress Is Associated with Increased Sensitivity to Neuropathic Pain and Sex-Specific Changes in Supraspinal MRNA Expression of Epigenetic- and Stress-Related Genes in Adulthood. Behav. Brain Res. 2020, 380, 112396. [Google Scholar] [CrossRef] [PubMed]
- Stephens, K.E.; Zhou, W.; Ji, Z.; Chen, Z.; He, S.; Ji, H.; Guan, Y.; Taverna, S.D. Sex Differences in Gene Regulation in the Dorsal Root Ganglion after Nerve Injury. BMC Genom. 2019, 20, 147. [Google Scholar] [CrossRef]
- Uddin, M.; Sipahi, L.; Li, J.; Koenen, K.C. Sex Differences in Dna Methylation May Contribute to Risk of PTSD and Depression: A Review of Existing Evidence. Depress Anxiety 2013, 30, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Chidambaran, V.; Zhang, X.; Martin, L.J.; Ding, L.; Weirauch, M.T.; Geisler, K.; Stubbeman, B.L.; Sadhasivam, S.; Ji, H. Dna Methylation at the Mu-1 Opioid Receptor Gene (OPRM1) Promoter Predicts Preoperative, Acute, and Chronic Postsurgical Pain after Spine Fusion. Pharm. Pers. Med. 2017, 10, 157–168. [Google Scholar] [CrossRef]
- Tammimäki, A.; Männistö, P.T. Catechol-O-Methyltransferase Gene Polymorphism and Chronic Human Pain: A Systematic Review and Meta-Analysis. Pharm. Genom. 2012, 22, 673–691. [Google Scholar] [CrossRef]
- Fageera, W.; Chaumette, B.; Fortier, M.È.; Grizenko, N.; Labbe, A.; Sengupta, S.M.; Joober, R. Association between COMT Methylation and Response to Treatment in Children with ADHD. J. Psychiatr. Res. 2021, 135, 86–93. [Google Scholar] [CrossRef]
- Liu, P.; Xing, B.; Chu, Z.; Liu, F.; Lei, G.; Zhu, L.; Gao, Y.; Chen, T.; Dang, Y.H. Dopamine D3 Receptor Knockout Mice Exhibit Abnormal Nociception in a Sex-Different Manner. J. Neurosci. Res. 2017, 95, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Funabashi, T.; Akema, T.; Kimura, F. Sex-Specific Differences in Pain Response by Dopamine in the Bed Nucleus of the Stria Terminalis in Rats. Neuroreport 2013, 24, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Mione, V.; Canterini, S.; Brunamonti, E.; Pani, P.; Donno, F.; Fiorenza, M.T.; Ferraina, S. Both the COMT Val158Met Single-Nucleotide Polymorphism and Sex-Dependent Differences Influence Response Inhibition. Front. Behav. Neurosci. 2015, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, F.; Erickson, L.; Liua, G.; Chena, J.; Weinberger, D.R. Effects of Sex and COMT Genotype on Environmentally Modulated Cognitive Control in Mice. Proc. Natl. Acad. Sci. USA 2012, 109, 20160–20165. [Google Scholar] [CrossRef] [PubMed]
- Tunbridge, E.M.; Harrison, P.J. Importance of the COMT Gene for Sex Differences in Brain Function and Predisposition to Psychiatric Disorders. Biol. Basis Sex Differ. Psychopharmacol. 2011, 8, 119–140. [Google Scholar] [CrossRef]
- Roberto, K.A.; Reynolds, S.G. Older Women’s Experiences with Chronic Pain: Daily Challenges and Self-Care Practices. J. Women Aging 2002, 14, 5–23. [Google Scholar] [CrossRef]
- Votaw, V.R.; McHugh, R.K.; Witkiewitz, K. Alcohol Use Disorder and Motives for Prescription Opioid Misuse: A Latent Class Analysis. Subst. Use Misuse 2019, 54, 1558–1568. [Google Scholar] [CrossRef]
- McLean, C.P.; Anderson, E.R. Brave Men and Timid Women? A Review of the Gender Differences in Fear and Anxiety. Clin. Psychol. Rev. 2009, 29, 496–505. [Google Scholar] [CrossRef]
- Peltier, M.R.; Sofuoglu, M.; Petrakis, I.L.; Stefanovics, E.; Rosenheck, R.A. Sex Differences in Opioid Use Disorder Prevalence and Multimorbidity Nationally in the Veterans Health Administration. J. Dual Diagn. 2021, 17, 124–134. [Google Scholar] [CrossRef]
- Moran, L.M.; Kowalczyk, W.J.; Phillips, K.A.; Vahabzadeh, M.; Lin, J.L.; Mezghanni, M.; Epstein, D.H.; Preston, K.L. Sex Differences in Daily Life Stress and Craving in Opioid-Dependent Patients. Am. J. Drug Alcohol Abus. 2018, 44, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cantero, M.T.; Blasco-Blasco, M.; Chilet-Rosell, E.; Peiró, A.M. Gender Bias in Therapeutic Effort: From Research to Health Care. Farm. Hosp. 2020, 44, 109–113. [Google Scholar] [PubMed]
- Schäfer, G.; Prkachin, K.M.; Kaseweter, K.A.; Williams, A.C.D.C. Health Care Providers’ Judgments in Chronic Pain: The Influence of Gender and Trustworthiness. Pain 2016, 157, 1618–1625. [Google Scholar] [CrossRef] [PubMed]



| Total n = 250 | Females n = 125 | Males n = 125 | |
|---|---|---|---|
| Age | 62 (14) | 64 (14) * | 59 (14) |
| Employment status (%) | |||
| At work | 10 | 10 | 10 |
| Retired | 59 | 68 | 52 |
| Work Disability | 31 | 22 | 38 |
| Diagnosis (%) | |||
| Nonspecific low back pain | 83 | 95 ** | 65 |
| Other pain | 17 | 5 | 35 |
| Pain intensity (0–100 mm) | 67 (21) | 68 (22) | 66 (20) |
| Relief (0–100 mm) | 32 (27) | 34 (26) | 30 (28) |
| Quality of life (0–100 mm) | 43 (23) | 40 (22) | 46 (23) |
| HAD-Anxiety (0–21 scores) | 8 [3, 12] | 9 [5, 13] * | 5 [2, 11] |
| HAD-Depression (0–21 scores) | 7 [4, 12] | 8 [5, 13] | 7 [3, 11] |
| MEDD (mg/day) | 106 (99) | 104 (99) | 109 (98) |
| Total Adverse Events | 3 [1, 6] | 3 [1, 6] | 3 [1, 5] |
| Opioid use disorder (%) | 21 | 15 | 26 * |
| Adverse Events (%) | |||
| Dry Mouth | 45 | 53 | 41 |
| Constipation | 41 | 46 | 42 |
| Insomnia | 28 | 34 | 26 |
| Dry Skin | 22 | 31 * | 16 |
| Nervousness | 26 | 30 | 26 |
| Dizziness | 26 | 32 | 23 |
| Sexual disturbance | 25 | 20 | 33 * |
| Weight changes | 23 | 33 * | 16 |
| Lack of appetite | 13 | 17 | 11 |
| Red skin | 11 | 27 | 13 |
| Code | CpG Sites | Total n = 250 | Female n = 125 | Male n = 125 | p-Value |
|---|---|---|---|---|---|
| OPRM1 DNA Methylation (%) | |||||
| CpG 1 | −32 | 8.2 (3.8) | 8.3 (3.6) | 8.1 (4.1) | 0.3 |
| CpG 2 | −18 | 16.6 (6.2) | 16.6 (5.8) | 16.7 (6.7) | 0.8 |
| CpG 3 | −14 | 14.2 (5.5) | 14.2 (5.0) | 14.2 (6.1) | 0.5 |
| CpG 4 | −10 | 10.1 (3.9) | 10.2 (3.5) | 10.0 (4.3) | 0.4 |
| CpG 5 | +12 | 8.3 (4.0) | 8.3 (3.5) | 8.3 (4.5) | 0.4 |
| COMT DNA Methylation (%) | |||||
| CpG 1 | −89 | 1.5 (1.0) | 1.5 (0.8) | 1.5 (1.1) | 0.1 |
| CpG 2 | −86 | 0.9 (1.0) | 0.8 (0.6) | 0.9 (1.2) | 0.1 |
| CpG 3 | −84 | 0.7 (0.9) | 0.7 (0.7) | 0.7 (1.1) | 0.05 |
| CpG 4 | −75 | 1.5 (0.9) | 1.5 (0.7) | 1.5 (1.1) | 0.3 |
| CpG 5 | −72 | 0.8 (0.9) | 0.7 (0.6) | 0.8 (1.1) | 0.06 |
| CpG 6 | −67 | 1.1 (1.5) | 1.1 (1.6) | 1 (1.4) | 0.07 |
| CpG 7 | −62 | 0.5 (0.8) | 0.5 (0.5) | 0.6 (1.0) | 0.1 |
| Estimate | SD | p-Value | |
|---|---|---|---|
| Pain intensity | |||
| OPRM1 | −0.079 | 0.250 | 0.751 |
| COMT | 0.717 | 1.114 | 0.520 |
| Sex | −0.521 | 3.023 | 0.863 |
| Relief | |||
| OPRM1 | 0.248 | 0.294 | 0.400 |
| COMT | −3.149 * | 1.344 | 0.020 |
| Sex | 3.326 | 3.65 | 0.363 |
| Quality of life | |||
| OPRM1 | 0.190 | 0.238 | 0.425 |
| COMT | −2.069 * | 1.028 | 0.046 |
| Sex | −2.108 | 2.83 | 0.457 |
| HAD-Anxiety | |||
| OPRM1 | −0.178 * | 0.088 | 0.046 |
| COMT | 0.228 | 0.273 | 0.404 |
| Sex | 1.869 * | 0.891 | 0.039 |
| HAD-Depression | |||
| OPRM1 | −0.072 | 0.081 | 0.378 |
| COMT | −0.011 | 0.251 | 0.965 |
| Sex | 0.78 | 0.821 | 0.345 |
| Opioid Use Disorder | |||
| OPRM1 | −0.165 ** | 0.036 | <0.001 |
| COMT | 0.018 | 0.104 | 0.859 |
| Sex | −2.123 ** | 0.772 | 0.006 |
| OPRM1: Sex | 0.099 * | 0.05 | 0.047 |
| MEDD (mg/day) | |||
| OPRM1 G-allele | −0.914 ** | 0.24 | <0.001 |
| OPRM1 | −0.023 ** | 0.008 | 0.005 |
| OPRM1: OPRM1 G-allele | 0.046 ** | 0.014 | 0.001 |
| Sex | 0.009 | 0.081 | 0.908 |
| Adverse Event | Estimate | SD | − Effect Prob. | + Effect Prob. |
|---|---|---|---|---|
| Constipation | ||||
| OPRM1 | 0.035 | 0.022 | 0 | 25.5 |
| COMT | −0.299 | 0.17 | 95.84 | 0.36 |
| Insomnia | ||||
| OPRM1 | 0.02 | 0.024 | 0.37 | 14.43 |
| COMT | −1.145 | 0.494 | 99.92 | 0 |
| Dry mouth | ||||
| OPRM1 | 0.009 | 0.022 | 0.32 | 2.99 |
| COMT | −0.416 | 0.215 | 98.66 | 0.08 |
| Dry skin | ||||
| OPRM1 | 0.006 | 0.026 | 3.66 | 8.88 |
| COMT | −0.648 | 0.381 | 98.16 | 0.37 |
| Lack of appetite | ||||
| OPRM1 | −0.03 | 0.035 | 42.15 | 2.37 |
| COMT | −1.191 | 0.636 | 98.89 | 0.44 |
| Red skin | ||||
| OPRM1 | 0.045 | 0.035 | 2.2 | 68.85 |
| COMT | −0.765 | 0.56 | 95.24 | 2.6 |
| Nervousness | ||||
| OPRM1 | −0.05 | 0.027 | 58.74 | 0.01 |
| COMT | −0.341 | 0.253 | 91.11 | 2.35 |
| Dizziness | ||||
| OPRM1 | −0.056 | 0.028 | 66.16 | 0 |
| COMT | 0.211 | 0.104 | 0.53 | 95.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agulló, L.; Muriel, J.; Margarit, C.; Escorial, M.; Garcia, D.; Herrero, M.J.; Hervás, D.; Sandoval, J.; Peiró, A.M. Sex Differences in Opioid Response Linked to OPRM1 and COMT genes DNA Methylation/Genotypes Changes in Patients with Chronic Pain. J. Clin. Med. 2023, 12, 3449. https://doi.org/10.3390/jcm12103449
Agulló L, Muriel J, Margarit C, Escorial M, Garcia D, Herrero MJ, Hervás D, Sandoval J, Peiró AM. Sex Differences in Opioid Response Linked to OPRM1 and COMT genes DNA Methylation/Genotypes Changes in Patients with Chronic Pain. Journal of Clinical Medicine. 2023; 12(10):3449. https://doi.org/10.3390/jcm12103449
Chicago/Turabian StyleAgulló, Laura, Javier Muriel, César Margarit, Mónica Escorial, Diana Garcia, María José Herrero, David Hervás, Juan Sandoval, and Ana M. Peiró. 2023. "Sex Differences in Opioid Response Linked to OPRM1 and COMT genes DNA Methylation/Genotypes Changes in Patients with Chronic Pain" Journal of Clinical Medicine 12, no. 10: 3449. https://doi.org/10.3390/jcm12103449