Endothelial Dysfunction Is Associated with Decreased Nitric Oxide Bioavailability in Dysglycaemic Subjects and First-Degree Relatives of Type 2 Diabetic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Primary and Secondary Endpoints
2.4. Laboratory Measurements
2.5. Endothelial Glycocalyx Assessment
2.6. Coronary Flow Reserve Assessment
2.7. Echocardiography
2.8. Two-Dimensional Strain Analysis
2.9. Statistical Analysis
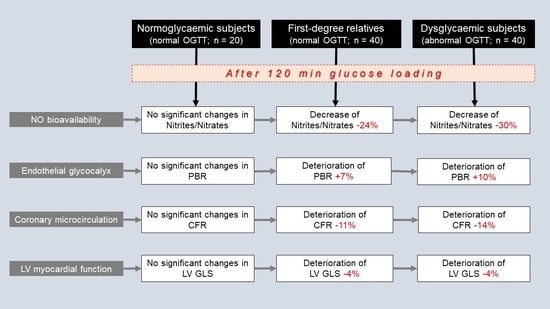
3. Results
3.1. Clinical and Biochemical Characteristics of the Three Study Groups
3.2. Oxidative Stress Markers and Nitrite/Nitrate Levels at Baseline and at 120 min after Glucose Loading
3.3. Vascular and Cardiac Markers at Baseline and at 120 min after Glucose Loading
3.4. Interrelation between Changes in Nitrite/Nitrate Levels and Changes in Vascular Markers
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambadiari, V.; Pavlidis, G.; Kousathana, F.; Maratou, E.; Georgiou, D.; Andreadou, I.; Kountouri, A.; Varoudi, M.; Balampanis, K.; Parissis, J.; et al. Effects of Different Antidiabetic Medications on Endothelial Glycocalyx, Myocardial Function, and Vascular Function in Type 2 Diabetic Patients: One Year Follow-Up Study. J. Clin. Med. 2019, 8, 983. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, S.R.; Roman, L.J.; Lamont, J.; Masters, B.S.; Bajaj, M.; Suraamornkul, S.; Belfort, R.; Berria, R.; Kellogg, D.L., Jr.; Liu, Y.; et al. Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J. Clin. Endocrinol. Metab. 2005, 90, 1100–1105. [Google Scholar] [CrossRef] [Green Version]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Thymis, J.; Simitsis, P.; Koliou, G.A.; Katsanos, S.; Triantafyllou, C.; Kousathana, F.; Pavlidis, G.; Kountouri, A.; Polyzogopoulou, E.; et al. Impaired Endothelial Glycocalyx Predicts Adverse Outcome in Subjects Without Overt Cardiovascular Disease: A 6-Year Follow-up Study. J. Cardiovasc. Transl. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Lekakis, J.; Abraham, P.; Balbarini, A.; Blann, A.; Boulanger, C.M.; Cockcroft, J.; Cosentino, F.; Deanfield, J.; Gallino, A.; Ikonomidis, I.; et al. Methods for evaluating endothelial function: A position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 775–789. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lambadiari, V.; Pavlidis, G.; Koukoulis, C.; Kousathana, F.; Varoudi, M.; Spanoudi, F.; Maratou, E.; Parissis, J.; Triantafyllidi, H.; et al. Insulin resistance and acute glucose changes determine arterial elastic properties and coronary flow reserve in dysglycaemic and first-degree relatives of diabetic patients. Atherosclerosis 2015, 241, 455–462. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Lambadiari, V.; Kousathana, F.; Varoudi, M.; Spanoudi, F.; Maratou, E.; Parissis, J.; Triantafyllidi, H.; Dimitriadis, G.; et al. Early detection of left ventricular dysfunction in first-degree relatives of diabetic patients by myocardial deformation imaging: The role of endothelial glycocalyx damage. Int. J. Cardiol. 2017, 233, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Cassano, V.; Miceli, S.; Armentaro, G.; Mannino, G.C.; Fiorentino, V.T.; Perticone, M.; Succurro, E.; Hribal, M.L.; Andreozzi, F.; Perticone, F.; et al. Oxidative Stress and Left Ventricular Performance in Patients with Different Glycometabolic Phenotypes. Nutrients 2022, 14, 1299. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Prevention of diabetes mellitus. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994, 844, 1–100. [Google Scholar]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Gutt, M.; Davis, C.L.; Spitzer, S.B.; Llabre, M.M.; Kumar, M.; Czarnecki, E.M.; Schneiderman, N.; Skyler, J.S.; Marks, J.B. Validation of the insulin sensitivity index (ISI(0,120)): Comparison with other measures. Diabetes Res. Clin. Pract. 2000, 47, 177–184. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Vlastos, D.; Andreadou, I.; Gazouli, M.; Efentakis, P.; Varoudi, M.; Makavos, G.; Kapelouzou, A.; Lekakis, J.; Parissis, J.; et al. Vascular conditioning prevents adverse left ventricular remodelling after acute myocardial infarction: A randomised remote conditioning study. Basic Res. Cardiol. 2021, 116, 9. [Google Scholar] [CrossRef]
- Ma, S.W.; Tomlinson, B.; Benzie, I.F. A study of the effect of oral glucose loading on plasma oxidant:antioxidant balance in normal subjects. Eur. J. Nutr. 2005, 44, 250–254. [Google Scholar] [CrossRef]
- Yadav, D.; Mishra, M.; Rana, S.; Subramani, S.K.; Prasad, G. Study of biochemical and oxidative stress markers in the first-degree relatives of persons with type 2 diabetes stratified by glucose tolerance test. Progr. Nutr. 2017, 19, 183–190. [Google Scholar]
- Konukoğlu, D.; Hatemi, H.; Ozer, E.M.; Gönen, S.; Akçay, T. The erythrocyte glutathione levels during oral glucose tolerance test. J. Endocrinol. Investig. 1997, 20, 471–475. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Salvadeo, S.A.; Ferrari, I.; Fogari, E.; Gravina, A.; Mereu, R.; Palumbo, I.; Maffioli, P.; Randazzo, S.; et al. Modification of vascular and inflammation biomarkers after OGTT in overweight healthy and diabetic subjects. Microvasc. Res. 2010, 79, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. NO generation from nitrite and its role in vascular control. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 915–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauer, T.; Preik, M.; Rassaf, T.; Strauer, B.E.; Deussen, A.; Feelisch, M.; Kelm, M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc. Natl. Acad. Sci. USA 2001, 98, 12814–12819. [Google Scholar] [CrossRef] [Green Version]
- Mah, E.; Noh, S.K.; Ballard, K.D.; Matos, M.E.; Volek, J.S.; Bruno, R.S. Postprandial hyperglycemia impairs vascular endothelial function in healthy men by inducing lipid peroxidation and increasing asymmetric dimethylarginine:arginine. J. Nutr. 2011, 141, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B.; Cohen, R.A. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am. J. Physiol. 1992, 263, H321–H326. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Stocklauser-Färber, K.; Rösen, P. Generation of reactive oxygen intermediates, activation of NF-kappaB, and induction of apoptosis in human endothelial cells by glucose: Role of nitric oxide synthase? Free Radic. Biol. Med. 1999, 27, 752–763. [Google Scholar] [CrossRef]
- Su, Y.; Liu, X.M.; Sun, Y.M.; Jin, H.B.; Fu, R.; Wang, Y.Y.; Wu, Y.; Luan, Y. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int. J. Clin. Pract. 2008, 62, 877–882. [Google Scholar] [CrossRef]
- Xiang, G.D.; Sun, H.L.; Hou, J.; Yue, L.; Xu, L. Acute hyperglycemia rapidly suppresses endothelium-dependent arterial dilation in first-degree relatives of type 2 diabetic patients. Exp. Clin. Endocrinol. Diabetes 2008, 116, 112–117. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.J.; Jeon, S.Y.; Hong, W.K.; Jung, S.E.; Kang, H.J.; Kim, J.W.; Jeon, J.P.; Han, B.G. Effect of glucose ingestion in plasma markers of inflammation and oxidative stress: Analysis of 16 plasma markers from oral glucose tolerance test samples of normal and diabetic patients. Diabetes Res. Clin. Pract. 2013, 99, e27–e31. [Google Scholar] [CrossRef]
- Esposito, K.; Ciotola, M.; Sasso, F.C.; Cozzolino, D.; Saccomanno, F.; Assaloni, R.; Ceriello, A.; Giugliano, D. Effect of a single high-fat meal on endothelial function in patients with the metabolic syndrome: Role of tumor necrosis factor-alpha. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Lambadiari, V.; Parissis, J.; Andreadou, I.; Tsoumani, M.; Boumpas, D.; Kouretas, D.; Iliodromitis, E. Tocilizumab improves oxidative stress and endothelial glycocalyx: A mechanism that may explain the effects of biological treatment on COVID-19. Food Chem. Toxicol. 2020, 145, 111694. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Aprea, C.; Albanese, G.; Di Martino, A.; Ricozzi, C.; et al. Can Metformin Exert as an Active Drug on Endothelial Dysfunction in Diabetic Subjects? Biomedicines 2020, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Caturano, A.; Galiero, R.; Di Martino, A.; Albanese, G.; Vetrano, E.; Sardu, C.; Marfella, R.; Rinaldi, L.; Sasso, F.C. Cardiovascular Benefits from Gliflozins: Effects on Endothelial Function. Biomedicines 2021, 9, 1356. [Google Scholar] [CrossRef]
- Takei, Y.; Tomiyama, H.; Tanaka, N.; Yamashina, A.; Chikamori, T. Association Between Insulin Resistance, Oxidative Stress, Sympathetic Activity and Coronary Microvascular Function in Patients with Early Stage Impaired Glucose Metabolism. Circ. J. 2022, 86, 866–873. [Google Scholar] [CrossRef]



| Normoglycaemic Subjects (n = 20) | First-Degree Relatives (n = 40) | Dysglycaemic Subjects (n = 40) | p-Value | |
|---|---|---|---|---|
| Age, years | 37 ± 8 | 39 ± 7 | 43 ± 8 | 0.410 |
| Sex (male/female), n (%) | 12/8 (60/40) | 22/18 (55/45) | 22/18 (55/45) | 0.833 |
| Systolic BP (mmHg) | 124 ± 14 | 127 ± 11 | 130 ± 9 | 0.116 |
| Diastolic BP (mmHg) | 77 ± 9 | 79 ± 8 | 83 ± 6 | 0.107 |
| Risk factors, n (%) | ||||
| Hypertension | 3 (15) | 6 (15) | 6 (15) | 0.260 |
| Dyslipidaemia | 8 (40) | 16 (40) | 17 (43) | 0.847 |
| Current smoking | 8 (40) | 17 (43) | 16 (40) | 0.893 |
| Family history CAD | 4 (20) | 7 (18) | 8 (20) | 0.972 |
| Metabolic characteristics | ||||
| BMI (kg/m2) | 28 ± 4 | 29 ± 5 | 30 ± 4 *** | 0.834 |
| Waist (cm) | 100 ± 13 | 101 ± 14 | 102 ± 12 | 0.259 |
| Hips (cm) | 101 ± 10 | 104 ± 12 | 106 ± 9 | 0.201 |
| Total cholesterol (mg/dL) | 185 ± 15 * | 214 ± 30 ** | 234 ± 26 ‡‡‡ | <0.001 |
| HDL cholesterol (mg/dL) | 58 ± 8 ‡ | 50 ± 8 ** | 46 ± 7 ‡‡‡ | <0.001 |
| LDL cholesterol (mg/dL) | 110 ± 15 * | 136 ± 23 ‡‡ | 155 ± 24 ‡‡‡ | <0.001 |
| Triglycerides (mg/dL) | 97 ± 22 ‡ | 136 ± 25 ** | 158 ± 28 ‡‡‡ | <0.001 |
| Fasting glucose (mg/dL) | 91 ± 11 | 95 ± 7 ‡‡ | 115 ± 26 ‡‡‡ | <0.001 |
| Glucose at 120 min (mg/dL) | 98 ± 15 * | 106 ± 16 ‡‡ | 200 ± 50 ‡‡‡ | <0.001 |
| Fasting insulin (μU/mL) | 8 ± 3 ‡ | 16 ± 8 | 15 ± 12 *** | 0.034 |
| Insulin at 120 min (μU/mL) | 29 ± 13 ‡ | 58 ± 33 ** | 79 ± 70 †† | 0.001 |
| Matsuda index | 5.5 ± 1.5 ‡ | 3 ± 1.2 | 2.9 ± 1.7 ‡‡‡ | <0.001 |
| ISI | 94.3 ± 17.1 † | 75.2 ± 19.6 ‡‡ | 39 ± 13.4 ‡‡‡ | <0.001 |
| Normoglycaemic Subjects (n = 20) | First-Degree Relatives (n = 40) | Dysglycaemic Subjects (n = 40) | |
|---|---|---|---|
| Malondialdehyde (nmol/L) | |||
| 0 min | 4.68 ± 1.9 | 4.79 ± 2.8 | 5.03 ± 2.1 |
| 120 min | 3.82 ± 1.7 | 4.07 ± 2.1 | 4.45 ± 2 |
| Δ% | −18 | −15 | −11 |
| Protein carbonyls (nmol/mL) | |||
| 0 min | 12.46 ± 4.9 | 11.15 ± 4.7 | 11.47 ± 5.3 |
| 120 min | 11.51 ± 5.8 | 10.71 ± 4.4 | 11.02 ± 5.6 |
| Δ% | −7 | −4 | −4 |
| Nitrites (μmol/L) | |||
| 0 min | 5.82 ± 1.9 | 5.96 ± 2.1 | 6.7 ± 2.6 |
| 120 min | 5.46 ± 1.3 | 4.57 ± 1.6 * | 4.2 ± 2 * |
| Δ% | −6 | −23 | −37 |
| Nitrates (μmol/L) | |||
| 0 min | 41.91 ± 16.3 | 44.39 ± 23.3 | 45.29 ± 18.9 |
| 120 min | 40.67 ± 16.5 | 33.9 ± 20.6 * | 32.36 ± 14.2 * |
| Δ% | −3 | −24 | −29 |
| Nitrites + Nitrates (μmol/L) | |||
| 0 min | 47.73 ± 15.9 | 50.35 ± 23.1 | 52.08 ± 19.7 |
| 120 min | 46.13 ± 16.4 | 38.47 ± 20.5 * | 36.57 ± 14.4 * |
| Δ% | −3 | −24 | −30 |
| PBR 20–25 μm | |||
| 0 min | 2.41 ± 0.3 | 2.5 ± 0.4 # | 2.52 ± 0.6 # |
| 120 min | 2.34 ± 0.3 | 2.67 ± 0.4 * | 2.77 ± 0.4 * |
| Δ% | −3 | +7 | +10 |
| CFR | |||
| 0 min | 3.17 ± 0.57 | 3.15 ± 0.4 | 2.79 ± 0.35 # |
| 120 min | 2.98 ± 0.56 | 2.81 ± 0.41 ‡ | 2.41 ± 0.31 ‡ |
| Δ% | −6 | −11 | −14 |
| LV GLS (%) | |||
| 0 min | −19.2 ± 2.1 | −18.4 ± 2.6 # | −16.8 ± 2 # |
| 120 min | −19.2 ± 2.4 | −17.6 ± 2.3 † | −16.2 ± 1.4 ‡ |
| Δ% | +0.1 | −4 | −4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikonomidis, I.; Pavlidis, G.; Tsoumani, M.; Kousathana, F.; Katogiannis, K.; Tsilivarakis, D.; Thymis, J.; Kountouri, A.; Korakas, E.; Pliouta, L.; et al. Endothelial Dysfunction Is Associated with Decreased Nitric Oxide Bioavailability in Dysglycaemic Subjects and First-Degree Relatives of Type 2 Diabetic Patients. J. Clin. Med. 2022, 11, 3299. https://doi.org/10.3390/jcm11123299
Ikonomidis I, Pavlidis G, Tsoumani M, Kousathana F, Katogiannis K, Tsilivarakis D, Thymis J, Kountouri A, Korakas E, Pliouta L, et al. Endothelial Dysfunction Is Associated with Decreased Nitric Oxide Bioavailability in Dysglycaemic Subjects and First-Degree Relatives of Type 2 Diabetic Patients. Journal of Clinical Medicine. 2022; 11(12):3299. https://doi.org/10.3390/jcm11123299
Chicago/Turabian StyleIkonomidis, Ignatios, George Pavlidis, Maria Tsoumani, Foteini Kousathana, Konstantinos Katogiannis, Damianos Tsilivarakis, John Thymis, Aikaterini Kountouri, Emmanouil Korakas, Loukia Pliouta, and et al. 2022. "Endothelial Dysfunction Is Associated with Decreased Nitric Oxide Bioavailability in Dysglycaemic Subjects and First-Degree Relatives of Type 2 Diabetic Patients" Journal of Clinical Medicine 11, no. 12: 3299. https://doi.org/10.3390/jcm11123299