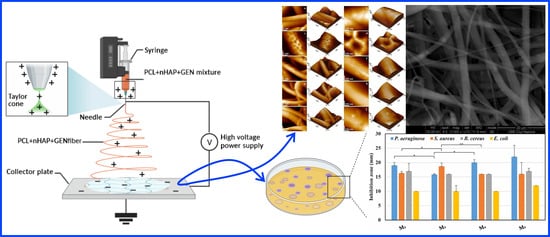
Morphology, Cytotoxicity, and Antimicrobial Activity of Electrospun Polycaprolactone Biomembranes with Gentamicin and Nano-Hydroxyapatite
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.1.1. Synthesis of nHAP and Preparation of the Electrospun NMs
2.1.2. Morphological Characterization of the Obtained NMs
2.1.3. Antibacterial Activity of the Obtained NMs
2.1.4. Cytotoxicity
2.1.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortellini, P.; Carnevale, G.; Sanz, M.; Tonetti, M.S. Treatment of deep and shallow intrabony defects A multicenter randomized controlled clinical trial. J. Clin. Periodontol. 1998, 25, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibres. Biomaterials 2005, 26, 4139–4147. [Google Scholar] [CrossRef]
- Dental Implants Market. Available online: https://www.researchandmarkets.com/reports/3934172/dental-implants-market-size-share-and-trends (accessed on 10 September 2021).
- Monje, A.; Chan, H.L.; Galindo-Moreno, P.; Elnayef, B.; Suarez-Lopez del Amo, F.; Wang, F.; Wang, H.L. Alveolar bone architecture: A systematic review and meta-analysis. J. Periodontol. 2015, 86, 1231–1248. [Google Scholar] [CrossRef]
- Bornsteinm, M.; Halbritter, S.; Harnisch, H.; Weberh, P.; Buser, D. A retrospective analysis of patients referred for implant placement to a specialty clinic: Indications, surgical procedures, and early failures. Int. J. Oral Maxillofac. Implants 2008, 23, 1109–1116. [Google Scholar]
- Clementini, M.; Morlupi, A.; Canullo, L.; Agrestini, C.; Bar-Lattani, A. Success rate of dental implants inserted in horizontal and vertical guided bone regenerated areas: A systematic review. Int. J. Oral Maxillofac. Surg. 2012, 41, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Jensens, S.; Terheyden, H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral Maxillofac. Implants 2009, 24, 218–236. [Google Scholar]
- Hammerle, C.H.; Jungr, E.; Feloutis, A. A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J. Clin. Periodontol. 2002, 29, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly(epsilon-capro-lactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Malik, R.; Garg, T.; Goyal, A.K.; Rath, G. Diacerein-loaded novel gastro retentive nanofiber system using PLLA: Development and in vitro characterization. Artif. Cells Nanomed. Biotechnol. 2016, 44, 928–936. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Ocak, H.; Kutuk, N.; Demetoglu, U.; Balcıoglu, E.; Ozdamar, S.; Alkan, A. Comparison of bovine bone-autogenic bone mixture versus platelet-rich fibrin for maxillary sinus grafting: Histologic and histomorphologic study. J. Oral Implantol. 2017, 43, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Horch, H.H.; Sader, R.; Pautke, C.; Neff, A.; Deppe, H.; Kolk, A. Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb) for bone regeneration in the reconstructive surgery of the jaws. Int. J. Oral Maxillofac. Surg. 2006, 35, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Best, S.M.; Bonfield, W.; Brooks, R.A.; Rushton, N.; Jayasinghe, S.N.; Edirisinghe, M.J. In vitro assessment of the biological response to nano-sized hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Wang, L.; Orme, C.A.; Bonstein, T.; Bush, P.J.; Nancollas, G.H. Dissolution at the nanoscale: Self-preservation of biominerals. Angew. Chem. Int. Ed. Engl. 2004, 43, 2697–2701. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable Scaffolds for Bone Regeneration Combined with Drug-Delivery Systems in Osteomyelitis Therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Mirică, I.C.; Furtos, G.; Lucaciu, O.; Păscuţa, P.; Vlassa, M.; Moldovan, M.; Câmpian, R.S. Electrospun membranes based on polycaprolactone, nano-Hydroxyapatite and metronidazole. Materials 2021, 14, 931. [Google Scholar] [CrossRef] [PubMed]
- Tisler, C.E.; Moldovan, M.; Petean, I.; Buduru, S.D.; Prodan, D.; Sarosi, C.; Leucuţa, D.-C.; Chifor, R.; Badea, M.E.; Ene, R. Human Enamel Fluorination Enhancement by Photodynamic Laser Treatment. Polymers 2022, 14, 2969. [Google Scholar] [CrossRef] [PubMed]
- Iosif, C.; Cuc, S.; Prodan, D.; Moldovan, M.; Petean, I.; Badea, M.E.; Sava, S.; Tonea, A.; Chifor, R. Effects of Acidic Environments on Dental Structures after Bracket Debonding. Int. J. Mol. Sci. 2022, 23, 15583. [Google Scholar] [CrossRef]
- ISO 10993: 2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Standardization Organization: Geneva, Switzerland, 2009; EN, Printed in Switzerland. pp. 5–9.
- Rogowska-Tylman, J.; Locs, J.; Salma, I.; Woźniak, B.; Pilmane, M.; Zalite, V.; Wojnarowicz, J.; Kędzierska-Sar, A.; Chudoba, T.; Szlązak, K.; et al. In vivo and in vitro study of a novel nanohydroxyapatite sonocoated scaffolds for enhanced bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 669–684. [Google Scholar] [CrossRef]
- Linh, N.V.L.; Du, N.T.; My, N.T.N.; Tuyen, N.N.; Phu, H.D.; Tram, N.X.T. Electrospun Polycaprolactone/Hydroxyapatite (PCL/HAp) microfibers as potential biomaterials for tissue engineering. Mater. Today Proc. 2022, 66, 2895–2899. [Google Scholar] [CrossRef]
- Rossi, R.; Carli, E.; Bambini, F.; Mummolo, S.; Licini, C.; Memè, L. The Use of Nano-Hydroxyapatite (NH) for Socket Preservation: Communication of an Upcoming Multicenter Study with the Presentation of a Pilot Case Report. Medicina 2023, 9, 1978. [Google Scholar] [CrossRef] [PubMed]
- Furtos, G.; Rivero, G.; Rapuntean, S.; Abraham, G.A. Amoxicillin-loaded Electrospun Nanocomposite Membranes for Dental Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 966–976. [Google Scholar] [CrossRef]
- Coimbra, P.; Freitas, J.P.; Gonçalves, T.; Gil, M.H.; Figueiredo, M. Preparation of gentamicin sulfate eluting fiber mats by emulsion and by suspension electrospinning. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 86. [Google Scholar] [CrossRef] [PubMed]
- Vedakumari, S.W.; Jancy, S.J.V.; Prabakaran, L.; Raja Pravin, Y.; Senthil, R. A review on background, process and application of electrospun nanofibers for tissue regeneration. Proc. Inst. Mech. Eng. H 2023, 237, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khodir, W.K.W.; Abdul Razak, A.H.; Ng, M.H.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Ramakrishna, S. Nano-featured scaffolds for tissue engineering: A re view of spinning methodologies. Tissue Eng. 2006, 12, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Robles, D.; Brizuela, A.; Fernández-Domínguez, M.; Gil, J. Osteoblastic and Bacterial Response of Hybrid Dental Implants. J. Funct. Biomater. 2023, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Corti, A.; Dorocka-Bobkowska, B.; Pompella, A. Positive Effects of UV-Photofunctionalization of Titanium Oxide Surfaces on the Survival and Differentiation of Osteogenic Precursor Cells—An In Vitro Study. J. Funct. Biomater. 2022, 13, 265. [Google Scholar] [CrossRef]
- Batool, S.A.; Ahmad, K.; Irfan, M.; Ur Rehman, M.A. Zn–Mn-Doped Mesoporous Bioactive Glass Nanoparticle-Loaded Zein Coatings for Bioactive and Antibacterial Orthopedic Implants. J. Funct. Biomater. 2022, 13, 97. [Google Scholar] [CrossRef]
- Moldovan, M.; Dudea, D.; Cuc, S.; Sarosi, C.; Prodan, D.; Petean, I.; Furtos, G.; Ionescu, A.; Ilie, N. Chemical and Structural Assessment of New Dental Composites with Graphene Exposed to Staining Agents. J. Funct. Biomater. 2023, 14, 163. [Google Scholar] [CrossRef]
- Mohamed, W.; Sommer, U.; Sethi, S.; Domann, E.; Thormann, U.; Schütz, I.; Lips, K.S.; Chakraborty, T.; Schnettler, R.; Alt, V. Intracellular proliferation of S. aureus inosteoblasts and effects of rifampicin and gentamicin on S. aureus intracellular proliferation and survival. Eur. Cell Mater. 2014, 28, 258–268. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 10, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Wiblin, R.T. Nosocomial pneumonia. In Prevention and Control of Nosocomial Infections, 3rd ed.; Wenzel, R.P., Ed.; Williams and Wilkins: Baltimore, MA, USA, 1997; pp. 807–819. [Google Scholar]
- Ferguson, T.E.; Reihill, J.A.; Walker, B.; Hamilton, R.A.; Martin, S.L. A Selective Irreversible Inhibitor of Furin Does Not Prevent Pseudomonas Aeruginosa Exotoxin A-Induced Airway Epithelial Cytotoxicity. PLoS ONE 2016, 11, e0159868. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O.; Mansouri, M.D.; Zakarevicz, D.; AlSharif, A.; Landon, G.C. In vivo efficacy of antimicrobial-coated devices. J. Bone Joint Surg. 2007, 89, 792e. [Google Scholar] [CrossRef]
- Min, J.; Braatz, R.D.; Hammond, P.T. Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials 2014, 35, 2507–2517. [Google Scholar] [CrossRef]
- Wojcik, M.; Kazimierczak, P.; Belcarz, A.; Wilczynska, A.; Vivcharenko, V.; Pajchel, L.; Adaszek, L.; Przekora, A. Biocompatible curdlan-based biomaterials loaded with gentamicin and Zn-doped nano-hydroxyapatite as promising dressing materials for the treatment of infected wounds and prevention of surgical site infections. Biomater. Adv. 2022, 139, 213006. [Google Scholar] [CrossRef]
- Kanak, N.A.; Shahruzzaman, M.; Islam, M.S.; Takafuji, M.; Rahman, M.M.; Kabir, S.F. Fabrication of Electrospun PLA-nHAp Nanocomposite for Sustained Drug Release in Dental and Orthopedic Applications. Materials 2023, 16, 3691. [Google Scholar] [CrossRef]






| No. | Code | Composition of the NMs | ||
|---|---|---|---|---|
| PCL (wt.%) | nHAP (wt.%) | GEN (wt.%) | ||
| 1 | M0 | 100 | 0 | 0 |
| 2 | M1 | 90 | 10 | 0 |
| 3 | M2 | 89.5 | 10 | 0.5 |
| 4 | M3 | 99 | 0 | 1 |
| 5 | M4 | 89 | 10 | 1 |
| 6 | M5 | 84 | 15 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirica, I.-C.; Furtos, G.; Moldovan, M.; Prodan, D.; Petean, I.; Campian, R.-S.; Pall, E.; Lucaciu, O. Morphology, Cytotoxicity, and Antimicrobial Activity of Electrospun Polycaprolactone Biomembranes with Gentamicin and Nano-Hydroxyapatite. Membranes 2024, 14, 10. https://doi.org/10.3390/membranes14010010
Mirica I-C, Furtos G, Moldovan M, Prodan D, Petean I, Campian R-S, Pall E, Lucaciu O. Morphology, Cytotoxicity, and Antimicrobial Activity of Electrospun Polycaprolactone Biomembranes with Gentamicin and Nano-Hydroxyapatite. Membranes. 2024; 14(1):10. https://doi.org/10.3390/membranes14010010
Chicago/Turabian StyleMirica, Ioana-Codruta, Gabriel Furtos, Marioara Moldovan, Doina Prodan, Ioan Petean, Radu-Septimiu Campian, Emoke Pall, and Ondine Lucaciu. 2024. "Morphology, Cytotoxicity, and Antimicrobial Activity of Electrospun Polycaprolactone Biomembranes with Gentamicin and Nano-Hydroxyapatite" Membranes 14, no. 1: 10. https://doi.org/10.3390/membranes14010010